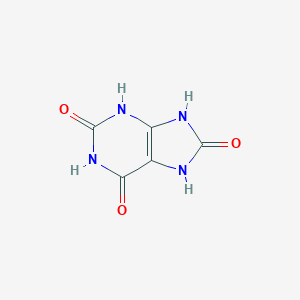
Details of Metabolite
| Full List of Protein(s) Regulating This Metabolite | ||||||
|---|---|---|---|---|---|---|
| Apolipoprotein (Apo) | ||||||
| Apolipoprotein A-II (APOA2) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] | ||||
| Introduced Variation | Mutation (-265T >C(rs5082)) of APOA2 | |||||
| Induced Change | Uric acid concentration: decrease (FC = 0.90) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Obesity [ICD-11: 5B81] | |||||
| Details | It is reported that mutation (-265T >C(rs5082)) of APOA2 leads to the decrease of uric acid levels compared with control group. | |||||
| Hydrolases (EC 3) | ||||||
| Leukotriene-C4 hydrolase (GGT1) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[2] | ||||
| Introduced Variation | Knockdown (siRNA) of GGT1 | |||||
| Induced Change | Uric acid concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Renal cell carcinoma [ICD-11: 2C90] | |||||
| Details | It is reported that knockdown of GGT1 leads to the increase of uric acid levels compared with control group. | |||||
| Isomerases (EC 5) | ||||||
| Alpha-methylacyl-CoA racemase (AMACR) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[3] | ||||
| Introduced Variation | Knockout of Amacr | |||||
| Induced Change | Uric acid concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that knockout of Amacr leads to the increase of uric acid levels compared with control group. | |||||
| Monocarboxylate porter (MNP) | ||||||
| Monocarboxylate transporter 10 (SLC16A10) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[4] | ||||
| Introduced Variation | Overexpression of SLC16A10 | |||||
| Induced Change | Uric acid concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that overexpression of SLC16A10 leads to the increase of uric acid levels compared with control group. | |||||
| Organic ion transporter (OIT) | ||||||
| Solute carrier family 22 member 13 (SLC22A13) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[5] | ||||
| Introduced Variation | Overexpression of SLC22A13 | |||||
| Induced Change | Uric acid concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that overexpression of SLC22A13 leads to the increase of uric acid levels compared with control group. | |||||
| Urate anion exchanger 1 (URAT1) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[6] | ||||
| Introduced Variation | Overexpression of Slc22a12 | |||||
| Induced Change | Uric acid concentration: increase (FC = 1.50) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Adrenomedullary hyperfunction [ICD-11: 5A75] | |||||
| Details | It is reported that overexpression of Slc22a12 leads to the increase of uric acid levels compared with control group. | |||||
| Pore-forming PNC peptide (PNC) | ||||||
| Cellular tumor antigen p53 (TP53) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[7] | ||||
| Introduced Variation | Knockout of TP53 | |||||
| Induced Change | Uric acid concentration: increase (Log2 FC=1.11) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Colon cancer [ICD-11: 2B90] | |||||
| Details | It is reported that knockout of TP53 leads to the increase of uric acid levels compared with control group. | |||||
| Sodium/anion cotransporter (SAC) | ||||||
| Sodium-dependent phosphate transport 4 (SLC17A3) | Click to Show/Hide the Full List of Regulating Pair(s): 3 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair (1) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[8] | ||||
| Introduced Variation | Mutation (F304S) of SLC17A3 | |||||
| Induced Change | Uric acid concentration: decrease | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that mutation (F304S) of SLC17A3 leads to the decrease of uric acid levels compared with control group. | |||||
| Regulating Pair (2) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[8] | ||||
| Introduced Variation | Mutation (N68H) of SLC17A3 | |||||
| Induced Change | Uric acid concentration: decrease | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that mutation (N68H) of SLC17A3 leads to the decrease of uric acid levels compared with control group. | |||||
| Regulating Pair (3) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[8] | ||||
| Introduced Variation | Overexpression of SLC17A3 | |||||
| Induced Change | Uric acid concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that overexpression of SLC17A3 leads to the increase of uric acid levels compared with control group. | |||||
| Sugar transporter (ST) | ||||||
| Glucose transporter type 9 (GLUT-9) | Click to Show/Hide the Full List of Regulating Pair(s): 2 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair (1) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[9] | ||||
| Introduced Variation | Overexpression of SLC2A9 | |||||
| Induced Change | Uric acid concentration: increase (FC = 31) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that overexpression of SLC2A9 leads to the increase of uric acid levels compared with control group. | |||||
| Regulating Pair (2) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[9] | ||||
| Introduced Variation | Inihibition (Benzbromarone) of SLC2A9 | |||||
| Induced Change | Uric acid concentration: decrease | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that inihibition of SLC2A9 leads to the decrease of uric acid levels compared with control group. | |||||
| Transcription factor (TF) | ||||||
| Forkhead box protein O1 (FOXO1) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[10] | ||||
| Introduced Variation | Overexpression of Foxo1 | |||||
| Induced Change | Uric acid concentration: increase (FC = 2.70) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that overexpression of Foxo1 leads to the increase of uric acid levels compared with control group. | |||||
| Transferases (EC 2) | ||||||
| Arylamine N-acetyltransferase 1 (NAT1) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[11] | ||||
| Introduced Variation | Knockout of NAT1 | |||||
| Induced Change | Uric acid concentration: increase (FC = 3.5) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Breast cancer [ICD-11: 2C60] | |||||
| Details | It is reported that knockout of NAT1 leads to the increase of uric acid levels compared with control group. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.