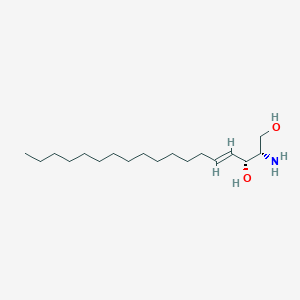
Details of Metabolite
| Full List of Protein(s) Regulating This Metabolite | ||||||
|---|---|---|---|---|---|---|
| Bone morphogenic antagonist (BMA) | ||||||
| DAN domain family member 5 (DAND5) | Click to Show/Hide the Full List of Regulating Pair(s): 2 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair (1) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] | ||||
| Introduced Variation | Knockdown (siRNA) of DAND5 | |||||
| Induced Change | Sphingosine concentration: decrease | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that knockdown of DAND5 leads to the decrease of sphingosine levels compared with control group. | |||||
| Regulating Pair (2) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] | ||||
| Introduced Variation | Knockdown (siRNA) of DAND5 | |||||
| Induced Change | Sphingosine concentration: increase (FC = 1.82) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that knockdown of DAND5 leads to the increase of sphingosine levels compared with control group. | |||||
| Hydrolases (EC 3) | ||||||
| Alkaline ceramidase 1 (ACER1) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[2] | ||||
| Introduced Variation | Knockout of Acer1 | |||||
| Induced Change | Sphingosine concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Healthy individual | |||||
| Details | It is reported that knockout of Acer1 leads to the increase of sphingosine levels compared with control group. | |||||
| Sulfatase sulf-1 (SULF1) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[3] | ||||
| Introduced Variation | Knockdown (shRNA) of SULF1 | |||||
| Induced Change | Sphingosine concentration: increase (FC = 5.08) | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | Ovarian cancer [ICD-11: 2C73] | |||||
| Details | It is reported that knockdown of SULF1 leads to the increase of sphingosine levels compared with control group. | |||||
| Transmembrane protein (TMEM) | ||||||
| ORM1-like protein 3 (ORMDL3) | Click to Show/Hide the Full List of Regulating Pair(s): 1 Pair(s) | |||||
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
|||||
| Regulating Pair |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[4] | ||||
| Introduced Variation | Knockout of Ormdl3 | |||||
| Induced Change | Sphingosine concentration: increase | |||||
| Summary | Introduced Variation
|
|||||
| Disease Status | White matter disorders [ICD-11: 8A45] | |||||
| Details | It is reported that knockout of Ormdl3 leads to the increase of sphingosine levels compared with control group. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.