| General Information of MET (ID: META00838) |
| Name |
Ergothioneine
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(2S)-3-(2-Mercapto-1H-imidazol-5-yl)-2-(trimethylammonio)propanoate; (2S)-3-(2-Mercapto-1H-imidazol-5-yl)-2-(trimethylammonio)propanoic acid; (AlphaS)-alpha-carboxy-2,3-dihydro-N,N,N-trimethyl-2-thioxo-1H-imidazole-4-ethanaminium inner salt; (S)-(1-Carboxy-2-(2-mercaptoimidazol-4-yl)ethyl)trimethylammonium hydroxide; 2 Thiol L histidine betaine; 2-Mercaptohistidine trimethylbetaine; 2-Thiol-L-histidine-betaine; 3-(2-Sulfanylidene-1,3-dihydroimidazol-4-yl)-2-trimethylammonio-propanoate; 3-(2-Sulfanylidene-1,3-dihydroimidazol-4-yl)-2-trimethylammonio-propanoic acid; 3-(2-Sulphanylidene-1,3-dihydroimidazol-4-yl)-2-trimethylammonio-propanoate; 3-(2-Sulphanylidene-1,3-dihydroimidazol-4-yl)-2-trimethylammonio-propanoic acid; Ergothioneine thiol; Ergothionine; Erythrothioneine; L-Ergothioneine; L-Thioneine; Sympectothion; Thiolhistidine-betaine; Thiolhistidinebetaine; Thioneine
|
| Source |
Food
|
| Structure Type |
Amino acids, peptides, and analogues (Click to Show/Hide the Complete Structure Type Hierarchy)
Organic acids and derivatives
Carboxylic acids and derivatives
Amino acids, peptides, and analogues
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
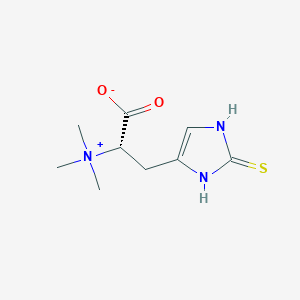
C9H15N3O2S
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5351619"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
229.3 |
Topological Polar Surface Area |
96.3 |
| XlogP |
0.3 |
Complexity |
314 |
| Heavy Atom Count |
15 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Function |
Ergothioneine is a naturally occurring metabolite of histidine that has antioxidant properties. -- Pubchem. Ergothioneine is a product of plant origin that accumulates in animal tissues. Ergothioneine is biosynthesized exclusively by fungi and mycobacteria and is captured by plants through their roots. As an ingredient of human food, ET is distributed very unevenly. By far, the highest levels of Ergothioneine have been found in mushrooms (0.1-1 mg/g dried material). Ergothioneine is rapidly cleared from the circulation and then avidly retained with minimal metabolism: the whole-body half-life of ingested Ergothioneine in rats is 1 month. The content of Ergothioneine varies greatly among tissues and is strongly dependent on its dietary level. In addition to erythrocytes and bone marrow, high Ergothioneine levels have also been found in seminal fluid. The precise physiological role of ET has remained elusive since its discovery in 1909. It is known that Ergothioneine is a powerful scavenger of hydroxyl radicals and an inhibitor of iron or copper ion-dependent generation of hydroxyl radicals from hydrogen peroxide (H2O2). A specific ergothioneine transporter has recently been identified (gene symbol SLC22A4 - PMID: 15795384). Ergothioneine appears to play a pivotal protective role in monocytes, because the occurrence of rheumatoid arthritis and Crohn's disease has very recently been linked to variant ergothioneine transporter genes. SLC22A4 is highly expressed in the kidney, where it is thought to aid in active secretion of organic cations, and may facilitate the active reabsorption of ergothioneine.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

