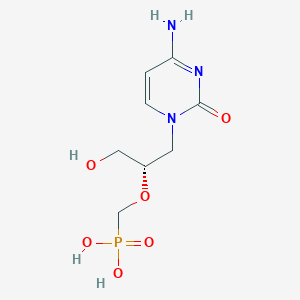
| Full List of Protein(s) Regulating This Metabolite |
| Organic ion transporter (OIT) |
|---|
| Organic anion transporter 1 (OAT1) |
Click to Show/Hide the Full List of Regulating Pair(s): 6 Pair(s)
|
| Detailed Information |
Protein Info
 click to show the details of this protein click to show the details of this protein
|
| Regulating Pair (1) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] |
| Introduced Variation |
Mutation (F438A) of SLC22A6 |
| Induced Change |
Cidofovir concentration: decrease (FC = 0.25) |
| Summary |
Introduced Variation
 Induced Change
Induced Change

|
| Disease Status |
Healthy individual
|
| Details |
It is reported that mutation (F438A) of SLC22A6 leads to the decrease of cidofovir levels compared with control group. |
| Regulating Pair (2) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] |
| Introduced Variation |
Mutation (F438Y) of SLC22A6 |
| Induced Change |
Cidofovir concentration: decrease (FC = 0.25) |
| Summary |
Introduced Variation

 Induced Change
Induced Change

|
| Disease Status |
Healthy individual
|
| Details |
It is reported that mutation (F438Y) of SLC22A6 leads to the decrease of cidofovir levels compared with control group. |
| Regulating Pair (3) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] |
| Introduced Variation |
Mutation (K431A) of SLC22A6 |
| Induced Change |
Cidofovir concentration: decrease (FC = 0.25) |
| Summary |
Introduced Variation

 Induced Change
Induced Change

|
| Disease Status |
Healthy individual
|
| Details |
It is reported that mutation (K431A) of SLC22A6 leads to the decrease of cidofovir levels compared with control group. |
| Regulating Pair (4) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] |
| Introduced Variation |
Mutation (K431R) of SLC22A6 |
| Induced Change |
Cidofovir concentration: decrease (FC = 0.25) |
| Summary |
Introduced Variation

 Induced Change
Induced Change

|
| Disease Status |
Healthy individual
|
| Details |
It is reported that mutation (K431R) of SLC22A6 leads to the decrease of cidofovir levels compared with control group. |
| Regulating Pair (5) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] |
| Introduced Variation |
Mutation (Y230A) of SLC22A6 |
| Induced Change |
Cidofovir concentration: decrease (FC = 0.25) |
| Summary |
Introduced Variation

 Induced Change
Induced Change

|
| Disease Status |
Healthy individual
|
| Details |
It is reported that mutation (Y230A) of SLC22A6 leads to the decrease of cidofovir levels compared with control group. |
| Regulating Pair (6) |
Experim Info
 click to show the details of experiment for validating this pair click to show the details of experiment for validating this pair
|
[1] |
| Introduced Variation |
Mutation (Y230F) of SLC22A6 |
| Induced Change |
Cidofovir concentration: decrease (FC = 0.25) |
| Summary |
Introduced Variation

 Induced Change
Induced Change

|
| Disease Status |
Healthy individual
|
| Details |
It is reported that mutation (Y230F) of SLC22A6 leads to the decrease of cidofovir levels compared with control group. |
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair