| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
3-Carboxypropylamine; 4 Aminobutanoic acid; 4 Aminobutyric acid; 4-Amino-butanoate; 4-Aminobutanoate; 4-Aminobutanoic acid; 4-Aminobutyrate; 4-Aminobutyric acid; 4Abu; Acid, hydrochloride gamma-aminobutyric; Aminalon; Aminalone; GABA; GABA, lithium; GAMMA-AMINO-butanoIC ACID; Gaballon; Gamarex; Gammalon; Gammalone; Gammar; Gammasol; Hydrochloride gamma-aminobutyric acid; Lithium gaba; Mielogen; Mielomade; Omega-aminobutyrate; Omega-aminobutyric acid; Piperidate; Piperidic acid; Piperidinate; Piperidinic acid; W-Aminobutyrate; W-Aminobutyric acid; g-AMINO-butanoate; g-AMINO-butanoic acid; g-Amino-N-butyrate; g-Amino-N-butyric acid; g-Aminobutanoate; g-Aminobutanoic acid; g-Aminobutyrate; g-Aminobutyric acid; gamma Aminobutyrate; gamma Aminobutyric acid; gamma Aminobutyric acid, hydrochloride; gamma Aminobutyric acid, monolithium salt; gamma Aminobutyric acid, monosodium salt; gamma-Amino-N-butyric acid; gamma-Aminobutanoic acid; gamma-Aminobuttersaeure; gamma-Aminobutyric acid; gamma-Aminobutyric acid, calcium salt (2:1); gamma-Aminobutyric acid, hydrochloride; gamma-Aminobutyric acid, monolithium salt; gamma-Aminobutyric acid, monosodium salt; gamma-Aminobutyric acid, zinc salt (2:1)
|
| Function |
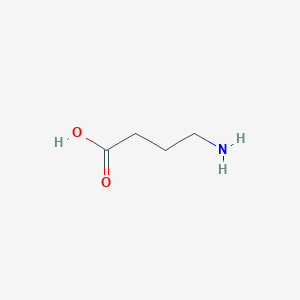
gamma-Aminobutyric acid (GABA) is an inhibitory neurotransmitter found in the nervous systems of widely divergent species, including humans. It is the chief inhibitory neurotransmitter in the vertebrate central nervous system. In vertebrates, GABA acts at inhibitory synapses in the brain. It acts by binding to specific transmembrane receptors in the plasma membrane of both pre- and postsynaptic neurons. This binding causes the opening of ion channels to allow either the flow of negatively-charged chloride ions into the cell or positively-charged potassium ions out of the cell. This will typically result in a negative change in the transmembrane potential, usually causing hyperpolarization. Three general classes of GABA receptor are known. These include GABA-A and GABA-C ionotropic receptors, which are ion channels themselves, and GABA-B metabotropic receptors, which are G protein-coupled receptors that open ion channels via intermediaries known as G proteins. Activation of the GABA-B receptor by GABA causes neuronal membrane hyperpolarization and a resultant inhibition of neurotransmitter release. In addition to binding sites for GABA, the GABA-A receptor has binding sites for benzodiazepines, barbiturates, and neurosteroids. GABA-A receptors are coupled to chloride ion channels. Therefore, activation of the GABA-A receptor induces increased inward chloride ion flux, resulting in membrane hyperpolarization and neuronal inhibition. After release into the synapse, free GABA that does not bind to either the GABA-A or GABA-B receptor complexes can be taken up by neurons and glial cells. Four different GABA membrane transporter proteins (GAT-1, GAT-2, GAT-3, and BGT-1), which differ in their distribution in the CNS, are believed to mediate the uptake of synaptic GABA into neurons and glial cells. The GABA-A receptor subtype regulates neuronal excitability and rapid changes in fear arousal, such as anxiety, panic, and the acute stress response. Drugs that stimulate GABA-A receptors, such as the benzodiazepines and barbiturates, have anxiolytic and anti-seizure effects via GABA-A-mediated reduction of neuronal excitability, which effectively raises the seizure threshold. GABA-A antagonists produce convulsions in animals and there is decreased GABA-A receptor binding in a positron emission tomography (PET) study of patients with panic disorder. Neurons that produce GABA as their output are called GABAergic neurons and have chiefly inhibitory action at receptors in the vertebrate. Medium spiny neurons (MSNs) are a typical example of inhibitory CNS GABAergic cells. GABA has been shown to have excitatory roles in the vertebrate, most notably in the developing cortex. Organisms synthesize GABA from glutamate using the enzyme L-glutamic acid decarboxylase and pyridoxal phosphate as a cofactor. It is worth noting that this involves converting the principal excitatory neurotransmitter (glutamate) into the principal inhibitory one (GABA). Drugs that act as agonists of GABA receptors (known as GABA analogs or GABAergic drugs), or increase the available amount of GABA typically have relaxing, anti-anxiety, and anti-convulsive effects. GABA is found to be deficient in cerebrospinal fluid and the brain in many studies of experimental and human epilepsy. Benzodiazepines (such as Valium) are useful in status epilepticus because they act on GABA receptors. GABA increases in the brain after administration of many seizure medications. Hence, GABA is clearly an antiepileptic nutrient. Inhibitors of GAM metabolism can also produce convulsions. Spasticity and involuntary movement syndromes, such as Parkinson's, Friedreich's ataxia, tardive dyskinesia, and Huntington's chorea, are all marked by low GABA when amino acid levels are studied. Trials of 2 to 3 g of GABA given orally have been effective in various epilepsy and spasticity syndromes. Agents that elevate GABA are also useful in lowering hypertension. Three grams orally have been effective in controlling blood pressure. GABA is decreased in various encephalopathies. GABA can reduce appetite and is decreased in hypoglycemics. GABA reduces blood sugar in diabetics. Chronic brain syndromes can also be marked by deficiencies of GABA. Vitamin B6, manganese, taurine, and lysine can increase both GABA synthesis and effects, while aspartic acid and glutamic acid probably inhibit GABA effects. Low plasma GABA has been reported in some depressed patients and may be a useful trait marker for mood disorders. GABA has an important role in embryonic development, especially facial development, as substantiated by the association of a cleft palate in transgenic mice deficient in GAD67 (glutamate decarboxylase). A recent Japanese population study reported linkage in patients with a nonsyndromic cleft lip with or without a cleft palate and specific GAD67 haplotypes. Unusually high levels of GABA (especially in the brain) can be toxic and GABA can function as both a neurotoxin and a metabotoxin. A neurotoxin is a compound that damages the brain and/or nerve tissue. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of GABA are associated with at least five inborn errors of metabolism, including D-2-hydroxyglutaric aciduria, 4-hydroxybutyric aciduria/succinic semialdehyde dehydrogenase deficiency, GABA-transaminase deficiency, homocarnosinosis, and hyper beta-alaninemia. Nearly all of these conditions are associated with seizures, hypotonia, intellectual deficits, macrocephaly, encephalopathy, and other serious neurological or neuromuscular problems. Increased levels of GABA seem to alter the function of the GABA-B receptor, which may play a role in the tonic-clonic seizures that are often seen in patients with the above disorders. GABA is also a microbial metabolite, urinary GABA is produced by Lactobacillus and Bifidobacterium.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair