| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
arachidonate; (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate; 506-32-1; CHEBI:32395; C20:4 fatty acid; (5Z,8Z,11Z,14Z)-icosatetraenoate; (5Z,8Z,11Z,14Z)-eicosatetraenoate; all-cis-icosa-5,8,11,14-tetraenoate; (20:4n6); A828211; (5Z,8Z,11Z,14Z)-eicosa-5,8,11,14-tetraenoate; (5Z,8Z,11Z,14Z)-icosatetra-5,8,11,14-enoate; Q27104215
|
| Function |
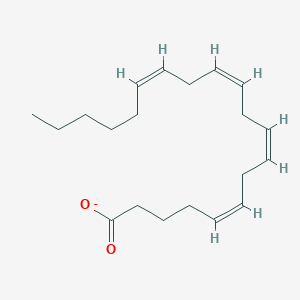
Arachidonic acid is a polyunsaturated, essential fatty acid that has a 20-carbon chain as a backbone and four cis-double bonds at the C5, C8, C11, and C14 positions. It is found in animal and human fat as well as in the liver, brain, and glandular organs, and is a constituent of animal phosphatides. It is synthesized from dietary linoleic acid. Arachidonic acid mediates inflammation and the functioning of several organs and systems either directly or upon its conversion into eicosanoids. Arachidonic acid in cell membrane phospholipids is the substrate for the synthesis of a range of biologically active compounds (eicosanoids) including prostaglandins, thromboxanes, and leukotrienes. These compounds can act as mediators in their own right and can also act as regulators of other processes, such as platelet aggregation, blood clotting, smooth muscle contraction, leukocyte chemotaxis, inflammatory cytokine production, and immune function. Arachidonic acid can be metabolized by cytochrome p450 (CYP450) enzymes into 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs), their corresponding dihydroxyeicosatrienoic acids (DHETs), and 20-hydroxyeicosatetraenoic acid (20-HETE). The production of kidney CYP450 arachidonic acid metabolites is altered in diabetes, pregnancy, hepatorenal syndrome, and in various models of hypertension, and it is likely that changes in this system contribute to the abnormalities in renal function that are associated with many of these conditions. Phospholipase A2 (PLA2) catalyzes the hydrolysis of the sn-2 position of membrane glycerophospholipids to liberate arachidonic acid. The beneficial effects of omega-3 fatty acids are believed to be due in part to selective alteration of arachidonate metabolism that involves cyclooxygenase (COX) enzymes. 9-Oxononanoic acid (9-ONA), one of the major products of peroxidized fatty acids, was found to stimulate the activity of phospholipase A2 (PLA2), the key enzyme to initiate the arachidonate cascade and eicosanoid production. Arachidonate lipoxygenase (ALOX) enzymes metabolize arachidonic acid to generate potent inflammatory mediators and play an important role in inflammation-associated diseases.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair