| General Information of MET (ID: META01230) |
| Name |
Esculin
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
Esculin; aesculin; 531-75-9; Esculoside; Esculin hydrate; (-)-Esculin; Polychrome; 6,7-Dihydroxycoumarin-6-O-glucoside; Bicolorin; Crataegin; Esculine; Esculetin 6-O-glucoside; 6,7-Dihydroxycoumarin 6-glucoside; UNII-1Y1L18LQAF; Vitamin C2; Esculetin 6-beta-D-glucoside; 6-(beta-D-Glucopyranosyloxy)-7-hydroxy-cumarin; CHEBI:4853; 7-Hydroxy-6-cumarinyl-glucosid; 6-(beta-D-Glucopyranosyloxy)-7-hydroxy-2H-1-benzopyran-2-one; 1Y1L18LQAF; Polychrom
|
| Source |
Aromatic heteropolycyclic compounds
|
| Structure Type |
Coumarin glycosides (Click to Show/Hide the Complete Structure Type Hierarchy)
Phenylpropanoids and polyketides
Coumarins and derivatives
Coumarin glycosides
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
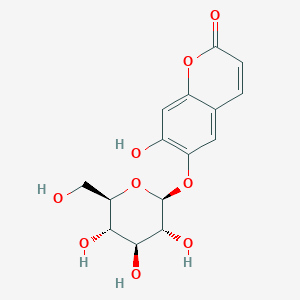
C15H16O9
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5281417"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
340.28 |
Topological Polar Surface Area |
146 |
| XlogP |
-0.6 |
Complexity |
495 |
| Heavy Atom Count |
24 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
5 |
Hydrogen Bond Acceptor Count |
9 |
| Function |
Esculin, also known as esculoside, belongs to the class of organic compounds known as coumarin glycosides. These are aromatic compounds containing a carbohydrate moiety glycosidically bound to a coumarin moiety. As a result, a total of 19 metabolites (10 phase I metabolites and 9 phase II metabolites) were found and identified. Esculin is an extremely weak basic (essentially neutral) compound (based on its pKa). The main activities of Esculine focus on capillary protection, as it improves capillary permeability and fragility. The antioxidant property might as well explain some of the anti-inflammatory activity of the product, making it a suitable product for after sun treatments, for example. After oral administration of esculin (100 mg/kg) for rats, plasma, urine, feces and bile samples were collected to screen metabolites. Esculine also showed good antioxidant properties, protecting triglycerides against auto-oxidation at high temperatures . Topically applied Esculine increases the capillary density (the number of capillaries open to flow per surface unit) and improves the morphological aspect of the smallest blood vessels. It was also found that after oral administration of esculin, the esculin could be metabolized to esculetin in vivo via deglycosylation, and esculetin was found in all biological samples. It is reported to inhibit catabolic enzymes such as hyaluronidase and collagenase, thus preserving the integrity of the perivascular connective tissue.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

