| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
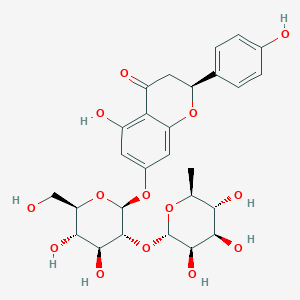
naringin; 10236-47-2; Naringoside; Naringin hydrate; UNII-N7TD9J649B; Naringenin 7-O-neohesperidoside; Naringenine-7-rhamnosidoglucoside; 4'5-diOH-Flavone-7-rhgluc; CHEMBL451532; N7TD9J649B; CHEBI:28819; Naringenin 7-Rhamnoglucoside; C27H32O14; MFCD00148888; aurantiin; Naringenin 7-O-[alpha-L-rhamnosyl-(1->2)-beta-D-glucoside]; (S)-7-(((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one; 7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one
|
| Function |
Naringin, also known as naringoside or naringin hydrate, belongs to the class of organic compounds known as flavonoid-7-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C7-position. It is metabolized to the flavonone naringenin in humans. Naringin is an extremely weak basic (essentially neutral) compound (based on its pKa). Naringin is a bitter tasting compound. Outside of the human body, Naringin is found, on average, in the highest concentration within a few different foods, such as rosemaries, grapefruit/pummelo hybrids, and grapefruits and in a lower concentration in grape wines, pummelo, and beers. Naringin has also been detected, but not quantified in, several different foods, such as citrus, limes, herbs and spices, common oregano, and mandarin orange (clementine, tangerine). This could make naringin a potential biomarker for the consumption of these foods. Both naringin and hesperetin, which are the aglycones of naringin and hesperidin, occur naturally in citrus fruits. Naringin is the major flavonoid glycoside in grapefruit and gives grapefruit juice its bitter taste. Narinigin exerts a variety of pharmacological effects such as antioxidant activity, blood lipid-lowering, anticarcinogenic activity, and inhibition of selected cytochrome P450 enzymes including CYP3A4 and CYP1A2, which may result in several drug interactions in-vitro.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair