| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
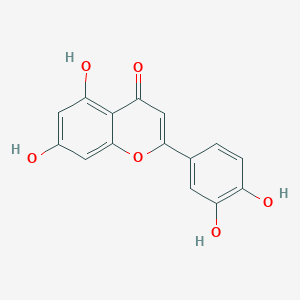
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-benzopyrone; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one', 3',4',5,7-Tetrahydroxyflavone, 5,7,3',4'-Tetrahydroxyflavone, 'Digitoflavone; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-benzopyrone-4-one; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one; Flacitran; Luteoline', 3',4',5,7-Tetrahydroxy-flavone; Luteolol; Salifazide
|
| Function |
Luteolin is a naturally occurring flavonoid. The flavonoids are polyphenolic compounds found as integral components of the human diet. They are universally present as constituents of flowering plants, particularly of food plants. The flavonoids are phenyl substituted chromones (benzopyran derivatives) consisting of a 15-carbon basic skeleton (C6-C3-C6), composed of a chroman (C6-C3) nucleus (the benzo ring A and the heterocyclic ring C), also shared by the tocopherols, with a phenyl (the aromatic ring B) substitution usually at the 2-position. Different substitutions can typically occur in the rings, A and B. Several plants and spices containing flavonoid derivatives have found application as disease preventive and therapeutic agents in traditional medicine in Asia for thousands of years. The selection of a particular food plant, plant tissue or herb for its potential health benefits appears to mirror its flavonoid composition. The much lower risk of colon, prostate and breast cancers in Asians, who consume more vegetables, fruits and tea than populations in the Western hemisphere do, raises the question of whether flavonoid components mediate the protective effects of diets rich in these foodstuffs by acting as natural chemopreventive and anticancer agents. An impressive body of information exists on the antitumoral action of plant flavonoids. In vitro work has concentrated on the direct and indirect actions of flavonoids on tumor cells, and has found a variety of anticancer effects such as cell growth and kinase activity inhibition, apoptosis induction, suppression of the secretion of matrix metalloproteinases and of tumor invasive behavior. Furthermore, some studies have reported the impairment of in vivo angiogenesis by dietary flavonoids. Experimental animal studies indicate that certain dietary flavonoids possess antitumoral activity. The hydroxylation pattern of the B ring of the flavones and flavonols, such as luteolin seems to critically influence their activities, especially the inhibition of protein kinase activity and antiproliferation. The different mechanisms underlying the potential anticancer action of plant flavonoids await further elucidation. Certain dietary flavonols and flavones targeting cell surface signal transduction enzymes, such as protein tyrosine and focal adhesion kinases, and the processes of angiogenesis appear to be promising candidates as anticancer agents. Further in vivo studies of these bioactive constituents is deemed necessary in order to develop flavonoid-based anticancer strategies. In view of the increasing interest in the association between dietary flavonoids and cancer initiation and progression, this important field is likely to witness expanded effort and to attract and stimulate further vigorous investigations.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair