|
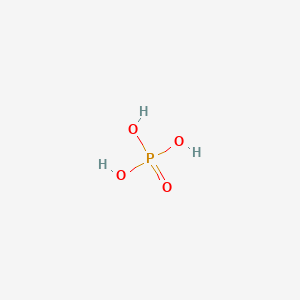
Phosphate is a salt of phosphoric acid and is an essential component of life. Organic phosphates are important in biochemistry, biogeochemistry, and ecology. In biological systems, phosphorus is found as a free phosphate ion in solution and is called inorganic phosphate, to distinguish it from phosphates bound in various phosphate esters. Inorganic phosphate is generally denoted Pi and at physiological (neutral) pH primarily consists of a mixture of HPO2-4 and H2PO-4 ions. Phosphates are most commonly found in the form of adenosine phosphates (AMP, ADP, and ATP) and in DNA and RNA, and can be released by the hydrolysis of ATP or ADP. Similar reactions exist for the other nucleoside diphosphates and triphosphates. Phosphoanhydride bonds in ADP and ATP, or other nucleoside diphosphates and triphosphates, contain high amounts of energy which give them their vital role in all living organisms. Phosphate must be actively transported into cells against its electrochemical gradient. In vertebrates, two unrelated families of Na+-dependent Pi transporters carry out this task. Remarkably, the two families transport different Pi species: whereas type II Na+/Pi cotransporters (SCL34) prefer divalent HPO4(2), type III Na+/Pi cotransporters (SLC20) transport monovalent H2PO4. The SCL34 family comprises both electrogenic and electroneutral members that are expressed in various epithelia and other polarized cells. Through regulated activity in apical membranes of the gut and kidney, they maintain body Pi homeostasis, and in salivary and mammary glands, liver, and testes they play a role in modulating the Pi content of luminal fluids. Phosphate levels in the blood play an important role in hormone signalling and in bone homeostasis. In classical endocrine regulation, low serum phosphate induces the renal production of the secosteroid hormone 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). This active metabolite of vitamin D acts to restore circulating mineral (i.e. phosphate and calcium) levels by increasing absorption in the intestine, reabsorption in the kidney, and mobilization of calcium and phosphate from bone. Thus, chronic renal failure is associated with hyperparathyroidism, which in turn contributes to osteomalacia (softening of the bones). Another complication of chronic renal failure is hyperphosphatemia (low levels of phosphate in the blood). Hyperphosphatemia (excess levels of phosphate in the blood) is a prevalent condition in kidney dialysis patients and is associated with increased risk of mortality. Hypophosphatemia (hungry bone syndrome) has been associated with postoperative electrolyte aberrations and after parathyroidectomy. Fibroblast growth factor 23 (FGF-23) has recently been recognized as a key mediator of phosphate homeostasis and its most notable effect is the promotion of phosphate excretion. FGF-23 was discovered to be involved in diseases such as autosomal dominant hypophosphatemic rickets, X-linked hypophosphatemia, and tumour-induced osteomalacia in which phosphate wasting was coupled to inappropriately low levels of 1,25(OH)2D3. FGF-23 is regulated by dietary phosphate in humans. In particular, it was found that phosphate restriction decreased FGF-23, and phosphate loading increased FGF-23. In agriculture, phosphate refers to one of the three primary plant nutrients, and it is a component of fertilizers. In ecological terms, because of its important role in biological systems, phosphate is a highly sought after resource. Consequently, it is often a limiting reagent in environments, and its availability may govern the rate of growth of organisms. Addition of high levels of phosphate to environments and to micro-environments in which it is typically rare can have significant ecological consequences. In the context of pollution, phosphates are a principal component of total dissolved solids, a major indicator of water quality. Dihydrogen phosphate is an inorganic salt used in numerous analytical methods, and in buffer solutions. It is one of several forms of the phosphate ion that can exist physiologically. It readily forms salts with sodium or potassium cations.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair