| General Information of MET (ID: META00759) |
| Name |
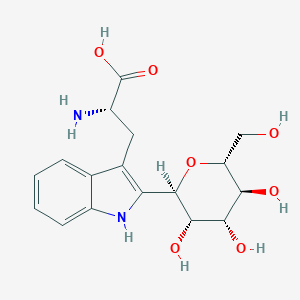
Tryptophan 2-C-mannoside
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
2-alpha-D-Mannopyranosyltryptophan; 2-alpha-D-Mannosyl-L-tryptophan; C-Glycosyltryptophan; C-Man-Trp; C-Mannosyl tryptophan; C-Mannosyltryptophan; L-Tryptophan 2-C-alpha-D-mannopyranoside; Tryptophan 2-C-mannoside
|
| Source |
Aromatic heteropolycyclic compounds
|
| Structure Type |
Indolyl carboxylic acids and derivatives (Click to Show/Hide the Complete Structure Type Hierarchy)
Organoheterocyclic compounds
Indoles and derivatives
Indolyl carboxylic acids and derivatives
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C17H22N2O7
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=10981970"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
366.4 |
Topological Polar Surface Area |
169 |
| XlogP |
-3.7 |
Complexity |
508 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
7 |
Hydrogen Bond Acceptor Count |
8 |
| Function |
Tryptophan 2-C-mannoside, also known as 2-alpha-D-mannopyranosyl-L-tryptophan or C-mannosyltryptophan, belongs to the class of organic compounds known as indolyl carboxylic acids and derivatives. Indolyl carboxylic acids and derivatives are compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an indole ring. It is an L-tryptophan derivative and a C-glycosyl compound in which the hydrogen at position 2 on the indole portion has been replaced by an alpha-mannosyl residue. Tryptophan 2-C-mannoside is a very strong basic compound (based on its pKa). Tryptophan 2-C-mannoside has been identified in blood and urine and is a marker of kidney function.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

