| General Information of MET (ID: META00723) |
| Name |
6-Oxopiperidine-2-carboxylic acid
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
6-OPCA; 6-Oxopiperidine-2-carboxylate; Adipo-2,6-lactam; Cyclic a-aminoadipate; Cyclic a-aminoadipic acid; Cyclic alpha-aminoadipic acid
|
| Structure Type |
Amino acids, peptides, and analogues (Click to Show/Hide the Complete Structure Type Hierarchy)
Organic acids and derivatives
Carboxylic acids and derivatives
Amino acids, peptides, and analogues
|
| PubChem CID |
|
| HMDB ID |
|
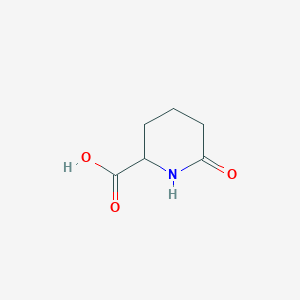
| Formula |
C6H9NO3
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=3014237"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| Physicochemical Properties |
Molecular Weight |
143.14 |
Topological Polar Surface Area |
66.4 |
| XlogP |
-0.4 |
Complexity |
166 |
| Heavy Atom Count |
10 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Function |
6-Oxopiperidine-2-carboxylic acid, also known as 6-Oxo-pipecolinic acid, or 6-Oxo-piperidine-2-carboxylic acid, is associated with penicillin V in the production on Penicillium chrysogenum fermentations. Analysis of a 13C NMR spectrum of a concentrated broth from Penicillium chrysogenum fermentation revealed the presence of penicillin V and 6-oxo-piperidine-2-carboxylic acid(1) as the principal constituents. The latter lactam, identical to an authentic sample prepared by the cyclization of alpha-aminoadipic acid was present to the extent of 28 mol% of penicillin V. The lactam isolated form the broth was nearly racemic, having a slight excess of the L-isomer. This isolation provides further evidence regarding the biosynthetic precursors of the hydrophobic penicillins. (PMID: 6788737
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

