| General Information of MET (ID: META00609) |
| Name |
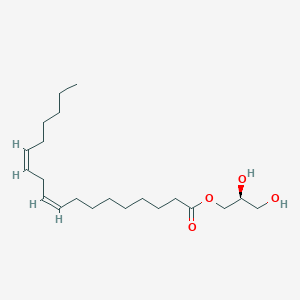
MG(18:2(9Z,12Z)/0:0/0:0)
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(S)-1-Monolinolein; (S)-1-O-Linoleoylglycerol; 1-(9Z,12Z)-Octadecadienoyl-sn-glycerol; 1-(9Z,12Z-Octadecadienoyl)-rac-glycerol; 1-(9Z,12Z-Octadecadienoyl)-sn-glycerol; 1-Glyceryl linoleate; 1-LG; 1-Linoleoyl-glycerol; 1-Linoleoyl-sn-monoglyceride; 1-Linoleylglycerol; 1-Monoacylglyceride; 1-Monoacylglycerol; 1-Monolinolein; 1-Monolinoleoyl-rac-glycerol; 1-O-(9Z,12Z-Octadecadienoyl)glycerol; 2,3-Dihydroxypropyl linoleate; 3-O-(9Z,12Z-Octadecadienoyl)glycerol; Glycerol 1-monolinolate; Glyceryl linoleic acid monoester; MAG(18:2); MAG(18:2/0:0); MAG(18:2W6/0:0); MAG(18:2n6/0:0); MG (18:2(N-6)/0:0/0:0); MG(18:2); MG(18:2/0:0); MG(18:2W6/0:0); MG(18:2n6/0:0); MLG CPD; Monolinolein; a-Monoacylglycerol; alpha-Glyceryl linoleate; alpha-Monoacylglycerol; sn-1-Monolinoleoylglycerol
|
| Source |
Endogenous;Food;Cosmetic;TCM Ingredients
|
| Structure Type |
Lineolic acids and derivatives (Click to Show/Hide the Complete Structure Type Hierarchy)
Lipids and lipid-like molecules
Fatty Acyls
Lineolic acids and derivatives
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C21H38O4
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=6436630"></iframe>
|
 |
|
3D MOL is unavailable
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
354.5 |
Topological Polar Surface Area |
66.8 |
| XlogP |
5.8 |
Complexity |
350 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
18 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Function |
MG(18:2(9Z,12Z)/0:0/0:0) is a monoacylglyceride. A monoglyceride, more correctly known as a monoacylglycerol, is a glyceride consisting of one fatty acid chain covalently bonded to a glycerol molecule through an ester linkage. Monoacylglycerol can be broadly divided into two groups; 1-monoacylglycerols (or 3-monoacylglycerols) and 2-monoacylglycerols, depending on the position of the ester bond on the glycerol moiety. Normally the 1-/3-isomers are not distinguished from each other and are termed 'alpha-monoacylglycerols', while the 2-isomers are beta-monoacylglycerols. Monoacylglycerols are formed biochemically via release of a fatty acid from diacylglycerol by diacylglycerol lipase or hormone sensitive lipase. Monoacylglycerols are broken down by monoacylglycerol lipase. They tend to be minor components only of most plant and animal tissues, and indeed would not be expected to accumulate because their strong detergent properties would have a disruptive effect on membranes. 2-Monoacylglycerols are a major end product of the intestinal digestion of dietary fats in animals via the enzyme pancreatic lipase. They are taken up directly by the intestinal cells and converted to triacylglycerols via the monoacylglycerol pathway before being transported in lymph to the liver. Mono- and Diglycerides are commonly added to commercial food products in small quantities. They act as emulsifiers, helping to mix ingredients such as oil and water that would not otherwise blend well.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

