| General Information of MET (ID: META00558) |
| Name |
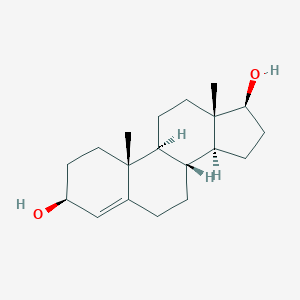
4-Androstenediol
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(3b,17b)-Androst-4-ene-3,17-diol; 3b,17b-Dihydroxy-4-androstene; 4-Androstene-3b,17b-diol; 4-Androstenediol; Androst-4-en-3b,17b-diol; Androst-4-ene-3beta,17beta-diol; D4-Androstene-3b,17b-diol
|
| Source |
Endogenous;Sterol lipids;Food;Drug
|
| Structure Type |
Androstane steroids (Click to Show/Hide the Complete Structure Type Hierarchy)
Lipids and lipid-like molecules
Steroids and steroid derivatives
Androstane steroids
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C19H30O2
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=136297"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
290.4 |
Topological Polar Surface Area |
40.5 |
| XlogP |
3.4 |
Complexity |
470 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
N.A. |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Function |
4-Androstenediol is a metabolite of testosterone. Conversely, the conversion of 4-androstenediol to testosterone has been demonstrated to occur in homogenates of hyperplastic human female adrenal glands. 4-Androstenediol is an anabolic agent that has been found in increased concentration in athletes suspected of doping. 4-Androstenediol has also been found in aqueous and solid nutritional supplements that are commercially available. Studies showing that non-hormonal supplements such as vitamins, minerals, and amino acids can contain anabolic androgenic steroids not declared on the labels of the products have been published. These undeclared substances (often prohormones of testosterone) can cause health risks to consumers and might lead to positive results in sports doping control. It has been demonstrated that 4-androstenediol taken by mouth is capable of producing in vivo increases in testosterone concentration in apparently healthy young men an women.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

