| General Information of MET (ID: META00504) |
| Name |
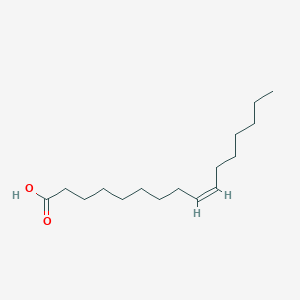
Palmitoleic acid
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(9Z)-9-Hexadecenoic acid; (9Z)-Hexadec-9-enoate; (9Z)-Hexadec-9-enoic acid; (9Z)-Hexadecenoate; (9Z)-Hexadecenoic acid; (Z)-9-Hexadecenoate; (Z)-9-Hexadecenoic acid; (Z)-Hexadec-9-enoate; (Z)-Hexadec-9-enoic acid; 16:1DElta9; 9-Hexadecenoate; 9-Hexadecenoic acid; 9-cis-Hexadecenoate; 9-cis-Hexadecenoic acid; 9Z-Hexadecenoic acid; C16:1; C16:1 trans-9; FA(16:1(9Z)); FA(16:1n7); Hexadecenoate; Hexadecenoate (N-C16:1); Hexadecenoic acid; Oleopalmitate; Oleopalmitic acid; Palmitelaidic acid; Palmitoleate; Palmitoleic acid; Palmitoleic acid, (Z)-isomer; Palmitoleic acid, (e)-isomer; Palmitolinoleate; Palmitolinoleic acid; Zoomarate; Zoomaric acid; Zoomerate; Zoomeric acid; cis-9-Hexadecenoate; cis-9-Hexadecenoic acid; cis-9-Palmitoleic acid; cis-Delta(9)-Hexadecenoic acid; cis-Palmitoleate; cis-Palmitoleic acid; cis-delta-9-Hexadecenoate; cis-delta-9-Hexadecenoic acid
|
| Source |
Endogenous;Escherichia Coli Metabolite;Yeast Metabolite;Fatty acyls;Food;TCM Ingredients;Microbial
|
| Structure Type |
Fatty acids and conjugates (Click to Show/Hide the Complete Structure Type Hierarchy)
Lipids and lipid-like molecules
Fatty Acyls
Fatty acids and conjugates
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C16H30O2
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=445638"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
254.41 |
Topological Polar Surface Area |
37.3 |
| XlogP |
6.4 |
Complexity |
209 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
13 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
2 |
| Function |
Palmitoleic acid, or 9-hexadecenoic acid, is an unsaturated fatty acid that is a common constituent of the glycerides of human adipose tissue. Present in all tissues, it is generally found in higher concentrations in the liver. Macadamia oil (Macadamia integrifolia) and sea buckthorn oil (Hippophae rhamnoides) are botanical sources of palmitoleic acid, containing 22 and 40% respectively. Palmitoleic acid is found to be associated with isovaleric acidemia, which is an inborn error of metabolism.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

