| General Information of MET (ID: META00499) |
| Name |
Flavone
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
2-PHENYL-4H-chromen-4-one; 2-Phenyl-4-benzopyron; 2-Phenyl-4-chromone; 2-Phenyl-4H-1-benzopyran-4-one; 2-Phenyl-4H-benzopyran-4-one; 2-Phenyl-gamma-benzopyrone; 2-Phenylbenzopyran-4-one; 2-Phenylchromone; Flavon; Flavone, 14C-labeled
|
| Source |
Food;Plant;Metabolite;Polyketides;Food;Drug;Toxins/Pollutant;TCM Ingredients;Plant Metabolite
|
| Structure Type |
Flavones (Click to Show/Hide the Complete Structure Type Hierarchy)
Phenylpropanoids and polyketides
Flavonoids
Flavones
|
| PubChem CID |
|
| HMDB ID |
|
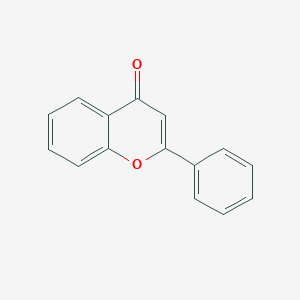
| Formula |
C15H10O2
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=10680"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
222.24 |
Topological Polar Surface Area |
26.3 |
| XlogP |
3.6 |
Complexity |
326 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
N.A. |
Hydrogen Bond Acceptor Count |
2 |
| Function |
Quercetin is a flavonoid that forms the "backbone" for many other flavonoids, including the citrus flavonoids rutin, hesperidin, naringin and tangeritin. In studies, quercetin is found to be the most active of the flavonoids, and many medicinal plants owe much of their activity to their high quercetin content. Quercetin has demonstrated significant anti-inflammatory activity because of direct inhibition of several initial processes of inflammation. For example, it inhibits both the manufacture and release of histamine and other allergic/inflammatory mediators. In addition, it exerts potent antioxidant activity and vitamin C-sparing action. -- Wikipedia.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

