| General Information of MET (ID: META00487) |
| Name |
Glycodeoxycholate sulfate
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
Glycoursodeoxycholic acid 3-sulfate; N-[(3a,5b,7b)-7-Hydroxy-24-oxo-3-(sulphooxy)cholan-24-yl]-glycine
|
| Source |
Endogenous;Sterol lipids;Food;Microbial
|
| Structure Type |
Bile acids, alcohols and derivatives (Click to Show/Hide the Complete Structure Type Hierarchy)
Lipids and lipid-like molecules
Steroids and steroid derivatives
Bile acids, alcohols and derivatives
|
| PubChem CID |
|
| HMDB ID |
|
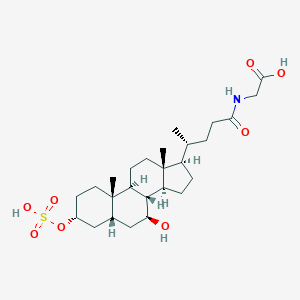
| Formula |
C26H43NO8S
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=21116917"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
529.7 |
Topological Polar Surface Area |
159 |
| XlogP |
3.9 |
Complexity |
950 |
| Heavy Atom Count |
36 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
8 |
| Function |
N-[(3a,5b,7b)-7-hydroxy-24-oxo-3-(sulfooxy)cholan-24-yl]-Glycine is an acyl glycine and a bile acid-glycine conjugate. It is a secondary bile acid produced by the action of enzymes existing in the microbial flora of the colonic environment. In hepatocytes, both primary and secondary bile acids undergo amino acid conjugation at the C-24 carboxylic acid on the side chain, and almost all bile acids in the bile duct therefore exist in a glycine conjugated form. Technically this compound is a sulfate conjugate of glycoursodeoxycholic acid.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

