|
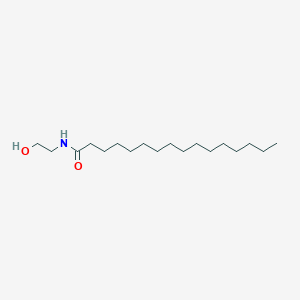
N-Palmitoylethanolamide (PEA) is present in the tissues of most mammals. It was initially described as an agonist of the type 2 cannabinoid receptor (CB2), although it is now universally recognized that PEA is in fact incapable of binding to cannabinoid receptors, or at least not to the known receptors. In addition to its anti-inflammatory activity, PEA also produces analgesia, neuroprotection, and possesses anti-epileptic properties. It also reduces gastrointestinal motility and cancer cell proliferation, as well as protecting the vascular endothelium in the ischemic heart. The physiological stimuli that regulate PEA levels in mammalian tissues are largely unknown, however, multiple studies indicate that this lipid accumulates during cellular stress, particularly following tissue injury. For example, PEA increases post-mortem in the pig brain. Similar elevations in PEA levels have been observed in the ischemic brain and PEA is also up-regulated in response to ultraviolet-B irradiation in mouse epidermal cells. Adipose tissue is highly implicated in the systemic secretion of IL-6 and leptin, and human mature adipocytes are able to secrete large quantity of PEA. Human adipose tissue can be subjected to modulation of its inflammatory state by lipopolysaccharide (LPS). LPS strongly inhibits adipose cell leptin release, with PEA acting as a potentiator of this inhibitory effect. These actions are not linked to a reduction in leptin gene transcription. Thus, PEA does not have an anti-inflammatory role in the secretion of IL-6 via NFkappaB at the adipocyte level, but instead seems to act at the heart of the LPS-stimulated pathway, which, independently of NFkappaB, inhibits the secretion of leptin.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair