| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
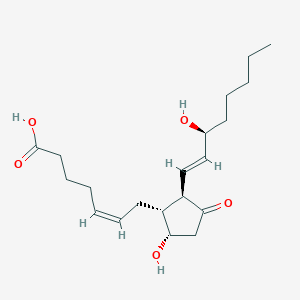
(5Z,13E)-(15S)-9,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,13E)-(15S)-9,15-Dihydroxy-11-oxoprosta-5,13-dienoic acid; (5Z,13E)-(15S)-9-alpha,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,13E)-(15S)-9-alpha,15-Dihydroxy-11-oxoprosta-5,13-dienoic acid; (5Z,13E)-(15S)-9a,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,13E)-(15S)-9a,15-Dihydroxy-11-oxoprosta-5,13-dienoic acid; (5Z,13E)-(15S)-9alpha,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,13E,15S)-9-alpha,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,13E,15S)-9-alpha,15-Dihydroxy-11-oxoprosta-5,13-dienoic acid; (5Z,13E,15S)-9a,15-Dihydroxy-11-oxoprosta-5,13-dien-1-Oate; (5Z,13E,15S)-9a,15-Dihydroxy-11-oxoprosta-5,13-dien-1-Oic acid; (5Z,13E,15S)-9a,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,13E,15S)-9a,15-Dihydroxy-11-oxoprosta-5,13-dienoic acid; (5Z,13E,15S)-9alpha,15-Dihydroxy-11-oxoprosta-5,13-dienoate; (5Z,9-alpha,13E,15S)-9,15-Dihydroxy-11-oxo-prosta-5,13-dien-1-Oate; (5Z,9-alpha,13E,15S)-9,15-Dihydroxy-11-oxo-prosta-5,13-dien-1-Oic acid; (5Z,9alpha,13E,15S)-9,15-Dihydroxy-11-oxo-prosta-5,13-dien-1-Oate; (5Z,9alpha,13E,15S)-9,15-Dihydroxy-11-oxo-prosta-5,13-dien-1-Oic acid; 11 Dehydroprostaglandin F2 alpha; 11 Dehydroprostaglandin F2alpha; 11-Dehydroprostaglandin F2 alpha; 11-Dehydroprostaglandin F2-alpha; 11-Dehydroprostaglandin F2alpha; 9S,15S-Dihydroxy-11-oxo-5Z,13E-prostadienoate; 9S,15S-Dihydroxy-11-oxo-5Z,13E-prostadienoic acid; D2, Prostaglandin; F2 alpha, 11-Dehydroprostaglandin; F2alpha, 11-Dehydroprostaglandin; PGD2; alpha, 11-Dehydroprostaglandin F2
|
| Function |
Prostaglandin D2 (or PGD2) is a prostaglandin that is actively produced in various organs such as the brain, spleen, thymus, bone marrow, uterus, ovary, oviduct, testis, prostate and epididymis, and is involved in many physiological events. PGD2 binds to the prostaglandin D2 receptor (PTGDR) which is a G-protein-coupled receptor. Its activity is mainly mediated by G-S proteins that stimulate adenylate cyclase resulting in an elevation of intracellular cAMP and Ca2+. PGD2 promotes sleep; regulates body temperature, olfactory function, hormone release, and nociception in the central nervous system; prevents platelet aggregation; and induces vasodilation and bronchoconstriction. PGD2 is also released from mast cells as an allergic and inflammatory mediator. Prostaglandin H2 is an unstable intermediate formed from PGG2 by the action of cyclooxygenase (COX) in the arachidonate cascade. In mammalian systems, it is efficiently converted into more stable arachidonate metabolites, such as PGD2, PGE2, PGF2a by the action of three groups of enzymes, PGD synthases (PGDS), PGE synthases and PGF synthases, respectively. PGDS catalyzes the isomerization of PGH2 to PGD2. Two types of PGD2 synthase are known. Lipocalin-type PGD synthase is present in cerebrospinal fluid, seminal plasma and may play an important role in male reproduction. Another PGD synthase, hematopoietic PGD synthase is present in the spleen, fallopian tube, endometrial gland cells, extravillous trophoblasts and villous trophoblasts, and perhaps plays an important role in female reproduction. Recent studies demonstrate that PGD2 is probably involved in multiple aspects of inflammation through its dual receptor systems, DP and CRTH2. Prostaglandins are eicosanoids. The eicosanoids consist of the prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and lipoxins (LXs). The PGs and TXs are collectively identified as prostanoids. Prostaglandins were originally shown to be synthesized in the prostate gland, thromboxanes from platelets (thrombocytes), and leukotrienes from leukocytes, hence the derivation of their names. All mammalian cells except erythrocytes synthesize eicosanoids. These molecules are extremely potent, able to cause profound physiological effects at very dilute concentrations. All eicosanoids function locally at the site of synthesis, through receptor-mediated G-protein linked signalling pathways.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair