| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
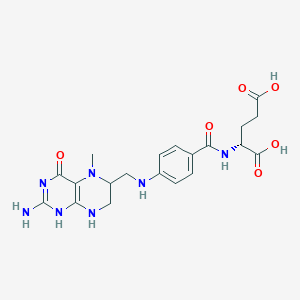
5-Methyl tetrahydrofolate; 5-Methyl-5,6,7,8-tetrahydrofolate; 5-Methyl-tetrahydrofolate; 5-Methyltetrahydrofolate; 5-Methyltetrahydrofolate, (DL-glu)-isomer; 5-Methyltetrahydrofolate, (L-glu)-(R)-isomer; 5-Methyltetrahydrofolate, (L-glu)-(S)-isomer; 5-Methyltetrahydrofolate, calcium salt (1:1), (L-glu)-isomer; 5-Methyltetrahydrofolate, methyl-(14)C-labeled, (DL-glu)-isomer; 5-Methyltetrahydrofolate, methyl-(14)C-labeled, (L-glu)-isomer; 5-Methyltetrahydropteroylglutamate; CH3-FH4; Deplin; L-Methyl folate; L-Methylfolate; Levomefolic acid; Mefolinate; Methyl folate; Methyl-tetrahydrofolate; N( 5)-Methyltetrahydrofolate; N(5)-Methyltetrahydrofolic acid; N-(4-(((2-Amino-1,4,5,6,7,8-hexahydro-5-methyl-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-L-glutamate; N-(4-(((2-Amino-1,4,5,6,7,8-hexahydro-5-methyl-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-L-glutamic acid; N-(5-Methyl-5,6,7,8-tetrahydropteroyl)-L-glutamate; N-(5-Methyl-5,6,7,8-tetrahydropteroyl)-L-glutamic acid; N5-Methyl-tetrahydrofolate; N5-Methyl-tetrahydrofolic acid; N5-Methyltetrahydrofolate; N5-Methyltetrahydropteroyl mono-L-glutamate; Prefolic a; [(6S)-5-Methyl-5,6,7,8-tetrahydropteroyl]glutamate
|
| Function |
5 methyltetrahydrofolic acid (5-MTHF) is the most biologically active form of the B-vitamin known as folic acid, also known generically as folate. 5-MTHF functions, in concert with vitamin B12, as a methyl-group donor involved in the conversion of the amino acid homocysteine to methionine. Methyl (CH3) group donation is vital to many bodily processes, including serotonin, melatonin, and DNA synthesis. Therapeutically, 5-MTHF is instrumental in reducing homocysteine levels, preventing neural tube defects, and improving vascular endothelial function. Research on folate supplementation suggests it plays a key role in preventing cervical dysplasia and protecting against neoplasia in ulcerative colitis. Folic acid also shows promise as part of a nutritional protocol to treat vitiligo, and may reduce inflammation of the gingiva. Furthermore, certain neurological, cognitive, and psychiatric presentations may be secondary to folate deficiency. Such presentations include depression, peripheral neuropathy, myelopathy, restless legs syndrome, insomnia, dementia, forgetfulness, irritability, endogenous depression, organic psychosis, and schizophrenia-like syndromes. After ingestion, the process of conversion of folic acid to the metabolically active coenzyme forms is relatively complex. Synthesis of the active forms of folic acid requires several enzymes, adequate liver and intestinal function, and adequate supplies of riboflavin (B2), niacin (B3), pyridoxine (B6), zinc, vitamin C, and serine. After formation of the coenzyme forms of the vitamin in the liver, these metabolically active compounds are secreted into the small intestine with bile (the folate enterohepatic cycle), where they are reabsorbed and distributed to tissues throughout the body. Human pharmacokinetic studies indicate folic acid has high bioavailability, with large oral doses of folic acid substantially raising plasma levels in healthy subjects in a time and dose dependent manner. Red blood cells (RBCs) appear to be the storage depot for folic acid, as RBC levels remain elevated for periods in excess of 40 days following discontinuation of supplementation. Folic acid is poorly transported to the brain and rapidly cleared from the central nervous system. The primary methods of elimination of absorbed folic acid are fecal (through bile) and urinary. Despite the biochemical complexity of this process, evidence suggests oral supplementation with folic acid increases the body's pool of 5-MTHF in healthy individuals. However, enzyme defects, mal-absorption, digestive system pathology, and liver disease can result in impaired ability to activate folic acid. In fact, some individuals have a severe congenital deficiency of the enzyme Methyl tetrahydrofolate reductase (5-MTHFR), which is needed to convert folic acid to 5-MTHF. Milder forms of this enzyme defect likely interact with dietary folate status to determine risk for some disease conditions. In individuals with a genetic defect of this enzyme (whether mild or severe), supplementation with 5- MTHF might be preferable to folic acid supplementation.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair