|
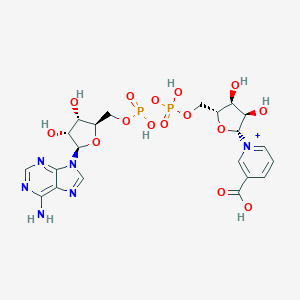
Nicotinic acid adenine dinucleotide, also known as deamido-NAD or NAAD, belongs to the class of organic compounds known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. NAAD is possibly soluble (in water) and a strong basic compound (based on its pKa). NAAD exists in all living species, ranging from bacteria to humans. L-Glutamine and NAAD can be converted into L-glutamic acid and NAD; which is catalyzed by the enzyme glutamine-dependent nad(+) synthetase. In humans, NAAD is involved in the nicotinate and nicotinamide metabolism pathway. NAAD is also involved in the metabolic disorder called succinic semialdehyde dehydrogenase deficiency. Outside of the human body, NAAD has been detected, but not quantified in, several different foods, such as japanese walnuts, cauliflowers, sparkleberries, komatsuna, and macadamia nut (m. tetraphylla). This could make NAAD a potential biomarker for the consumption of these foods. NAAD is the product of the degradation of Nicotinic acid adenine dinucleotide phosphate (NAADP) by a Ca2+-sensitive phosphatase. NAADP is a Ca2+-mobilizing second messenger which is synthesized, in response to extracellular stimuli, via the base-exchange reaction by an ADP-ribosyl cyclase (ARC) family members (such as CD38). NAADP binds to and opens Ca2+ channels on intracellular organelles, thereby increasing the intracellular Ca2+ concentration which, in turn, modulates a variety of cellular processes. Structurally, NAADP it is a dinucleotide that only differs from the house-keeping enzyme cofactor, NADP, by a hydroxyl group (replacing the nicotinamide amino group) and yet this minor modification converts it into the most potent Ca2+-mobilizing second messenger yet described. NAADP may also be broken down to 2-phosphoadenosine diphosphoribose (ADPRP) by CD38 or reduced to NAADPH.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair