| General Information of MET (ID: META00373) |
| Name |
2-Aminobenzoic acid
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
1-Amino-2-carboxybenzene; 2-Amino-benzoate; 2-Amino-benzoic acid; 2-Aminobenzoate; 2-Aminobenzoesaeure; 2-Aminophenylacetate; 2-Aminophenylacetic acid; 2-Carboxyaniline; Anthranate; Anthranic acid; Anthranilate; Anthranilic acid; Anthranilic acid GR; Anthranilic acid, cadmium salt; Anthranilic acid, calcium (2:1) salt; Anthranilic acid, dihydrochloride; Anthranilic acid, hydrochloride; Anthranilic acid, monolithium salt; Anthranilic acid, monosodium salt; Carboxyaniline; H-2-Abz-OH; Kyselina O-aminobenzoova; Kyselina anthranilova; O-Amino-benzoate; O-Amino-benzoic acid; O-Aminobenzoate; O-Aminobenzoesaeure; O-Aminobenzoic acid; O-Anthranilate; O-Anthranilic acid; O-Carboxyaniline; Ortho-amidobenzoate; Ortho-amidobenzoic acid; Ortho-aminobenzoate; Ortho-aminobenzoic acid; Sodium anthranilate; Vitamin L; Vitamin L1
|
| Source |
Food;Escherichia Coli Metabolite;Yeast Metabolite;Food;Carcinogenic Potency;Drug;Toxins/Pollutant;TCM Ingredients;Microbial
|
| Structure Type |
Benzoic acids and derivatives (Click to Show/Hide the Complete Structure Type Hierarchy)
Benzenoids
Benzene and substituted derivatives
Benzoic acids and derivatives
|
| PubChem CID |
|
| HMDB ID |
|
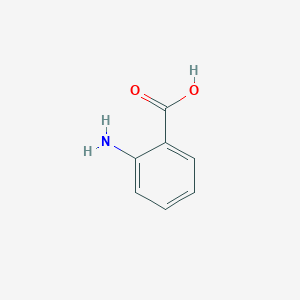
| Formula |
C7H7NO2
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=227"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
137.14 |
Topological Polar Surface Area |
63.3 |
| XlogP |
1.2 |
Complexity |
136 |
| Heavy Atom Count |
10 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Function |
2-Aminobenzoic acid is an organic compound. It is a substrate of enzyme anthranilate hydroxylase [EC 1.14.13.35] in benzoate degradation via hydroxylation pathway (KEGG). 2-Aminobenzoic acid has been identified as a uremic toxin according to the European Uremic Toxin Working Group.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

