| General Information of MET (ID: META00335) |
| Name |
N-alpha-Acetyl-L-citrulline
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(2S)-2-(Acetylamino)-5-[(aminocarbonyl)amino]pentanoate; (2S)-2-(Acetylamino)-5-[(aminocarbonyl)amino]pentanoic acid; (S)-2-ACETAMIDO-5-ureidopentanoIC ACID; (S)-2-ACETAMIDO-5-ureidopentanoate; N-Acetyl-L-citrulline; N2-Acetyl-N5-(aminocarbonyl)-L-ornithine; Nalpha-acetyl-L-citrulline
|
| Source |
Endogenous;Food
|
| Structure Type |
Amino acids, peptides, and analogues (Click to Show/Hide the Complete Structure Type Hierarchy)
Organic acids and derivatives
Carboxylic acids and derivatives
Amino acids, peptides, and analogues
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C8H15N3O4
|
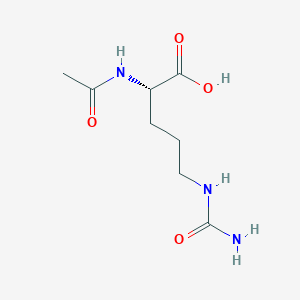
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=656979"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
217.22 |
Topological Polar Surface Area |
122 |
| XlogP |
-2.3 |
Complexity |
254 |
| Heavy Atom Count |
15 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
4 |
| Function |
N-alpha-Acetyl-L-citrulline, also known as N-acetylcitrulline, is an N-acetylated metabolite of citrulline that is part of the arginine biosynthetic pathway. Arginine biosynthesis is notable for its complexity and variability at the genetic level, and by its connection with several other pathways, such as pyrimidine and polyamine biosynthesis, and certain degradative pathways. The initial steps of the arginine biosynthetic pathways proceed via N-acetylated intermediates. The presumed reason for this is that the acetylation prevents the spontaneous cyclization of glutamate derivatives, which leads to proline biosynthesis. N-acetyl-L-ornithine can be transcarbamylated directly by the enzyme acetylornithine transcarbamylase, resulting in N-acetyl-L-citrulline. The enzyme acetylornithine deacetylase can accept N-acetyl-L-citrulline as a substrate and can deacetylate it into citrulline. N-alpha-Acetyl-L-citrulline is found in cases of deficiency of the urea cycle enzyme argininosuccinate synthase (EC 6.3.4.5) that leads to increased concentrations of citrulline and N-acetylcitrulline in the urine.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

