| General Information of MET (ID: META00258) |
| Name |
Allantoin
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(2,5-Dioxo-4-imidazolidinyl)urea; (S)-Allantoin; 2,5-Dioxo-4-imidazolidinyl-urea; 4-Ureido-2,5-imidazolidinedione; 5-Ureido-2,4-imidazolidindione; 5-Ureido-hydantoin; 5-Ureidohydantoin; 5-Ureidohydrantoin; AVC/Dienestrolcream; Alantan; Allantoin campbell brand; Allantol; Alloxantin; Campbell brand OF allantoin; Cordianine; D00121; Fancol toin; Glyoxyldiureid; Glyoxyldiureide; Glyoxylic diureide; Herpecin L; Herpecin-L; HerpecinL; N-(2,5-Dioxo-4-imidazolidinyl)urea; Psoralon; Reed and carnrick brand OF allantoin', Woun'dres, 'Allantoin sween brand; Sebical; Septalan; Sween brand OF allantoin
|
| Source |
Endogenous;Escherichia Coli Metabolite;Yeast Metabolite;Food;Carcinogenic Potency;Toxins/Pollutant;Cosmetic;TCM Ingredients;Microbial
|
| Structure Type |
Imidazoles (Click to Show/Hide the Complete Structure Type Hierarchy)
Organoheterocyclic compounds
Azoles
Imidazoles
|
| PubChem CID |
|
| HMDB ID |
|
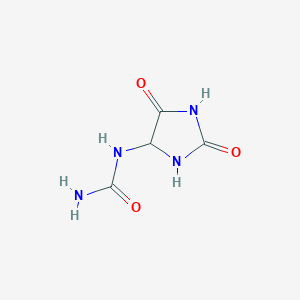
| Formula |
C4H6N4O3
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=204"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
158.12 |
Topological Polar Surface Area |
113 |
| XlogP |
-2.2 |
Complexity |
225 |
| Heavy Atom Count |
11 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
3 |
| Function |
Allantoin is a diureide of glyoxylic acid with the chemical formula C4H6N4O3. It is also called 5-ureidohydantoin or glyoxyldiureide. It is a product of the oxidation of uric acid. It is also a product of purine metabolism in most mammals except for higher apes, and it is present in their urine. In humans, uric acid is excreted instead of allantoin. The presence of allantoin in the urine can be an indication of microbial overgrowth or it can be created via non-enzymatic means through high levels of reactive oxygen species. In this regard, allantoin is sometimes used as a marker of oxidative stress. Allantoin can be isolated from cow urine or as a botanical extract of the comfrey plant. It has long been used for its healing, soothing, and anti-irritating properties. Allantoin helps to heal wounds and skin irritations and stimulates the growth of healthy tissue. Allantoin can be found in anti-acne products, sun care products, and clarifying lotions because of its ability to help heal minor wounds and promote healthy skin. Allantoin is frequently present in toothpaste, mouthwash, and other oral hygiene products as well as in shampoos, lipsticks, various cosmetic lotions and creams, and other cosmetic and pharmaceutical products. It is also a metabolite of Bacillus and Streptomyces.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

