| General Information of MET (ID: META00257) |
| Name |
L-alpha-Aminobutyric acid
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(+)-2-Aminobutanoate; (+)-2-Aminobutanoic acid; (+)-2-Aminobutyric acid; (+)-alpha-Aminobutyric acid; (-)-2-Aminobutyrate; (-)-2-Aminobutyric acid; (2S)-2-Aminobutanoate; (2S)-2-Aminobutanoic acid; (2S)-2-Aminobutyrate; (2S)-2-Aminobutyric acid; (S)-(+)-alpha-Aminobutyric acid; (S)-2-Amino-butanoate; (S)-2-Amino-butanoic acid; (S)-2-Aminobutanoate; (S)-2-Aminobutanoic acid; (S)-2-Aminobutyrate; (S)-2-Aminobutyric acid; 2-Aminobutanoate; 2-Aminobutanoic acid; 2-Aminobutyrate; 2-Aminobutyric acid; 2S-Amino-butanoate; 2S-Amino-butanoic acid; Butyrine; Butyrine, (+-)-isomer; Butyrine, (R)-isomer; Butyrine, (S)-isomer; Homoalanine; L-(+)-2-Aminobutyrate; L-(+)-2-Aminobutyric acid; L-2-Amino-N-butyric acid; L-2-Aminobutanoate; L-2-Aminobuttersaeure; L-2-Aminobutyrate; L-2-Aminobutyric acid; L-Butyrine; L-Ethylglycine; L-Homoalanine; L-a-Amino-N-butyrate; L-a-Amino-N-butyric acid; L-a-Aminobutyrate; L-a-Aminobutyric acid; L-alpha-Amino-N-butyric acid; S-Butyrine; alpha-Aminobutyric acid; alpha-Aminobutyric acid, (+-)-isomer; alpha-Aminobutyric acid, (R)-isomer; alpha-Aminobutyric acid, (S)-isomer
|
| Source |
Endogenous;Yeast Metabolite;Fatty acyls;Food;Microbial
|
| Structure Type |
Amino acids, peptides, and analogues (Click to Show/Hide the Complete Structure Type Hierarchy)
Organic acids and derivatives
Carboxylic acids and derivatives
Amino acids, peptides, and analogues
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C4H9NO2
|
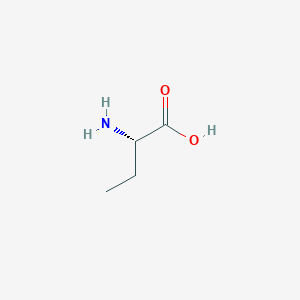
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=80283"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
103.12 |
Topological Polar Surface Area |
63.3 |
| XlogP |
-2.5 |
Complexity |
72.1 |
| Heavy Atom Count |
7 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Function |
L-alpha-Aminobutyric acid, also known as (S)-2-aminobutanoic acid, homoalanine, 2-AABA, ethylglycine, or L-butyrine, is a member of the class of compounds known as L-alpha-amino acids. L-alpha-Amino acids are alpha amino acids which have the L-configuration of the alpha-carbon atom. L-alpha-aminobutyric acid is soluble (in water) and is a moderately acidic compound (based on its pKa). L-alpha-Aminobutyric acid is a non-proteogenic amino acid that can be found in the human kidney, in liver tissues, and in most biofluids or excreta (e.g. feces, breast milk, urine, and blood). Within the cell, L-alpha-aminobutyric acid is primarily located in the cytoplasm. alpha-Aminobutyric acid is biosynthesized by transaminating oxobutyrate, a metabolite in isoleucine biosynthesis. As a non-proteogenic amino acid, alpha-aminobutyric acid can be used by nonribosomal peptide synthases. One example of a nonribosomal peptide containing alpha-aminobutyric acid is ophthalmic acid, which was first isolated from calf lens. alpha-Aminobutyric acid is a non-essential amino acid that is primarily derived from the catabolism of methionine, threonine, and serine. High protein diets can result in significantly higher alpha-aminobutyrate levels in plasma. alpha-Aminobutyric acid is elevated in the plasma of children with Reye's syndrome, tyrosinemia, homocystinuria, nonketotic hyperglycinemia, and ornithine transcarbamylase deficiency. alpha-Aminobutyric acid is one of the three isomers of aminobutyric acid. The two other are the neurotransmitter gamma-aminobutyric acid (GABA) and beta-aminobutyric acid (BABA) which is known for inducing plant disease resistance.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

