|
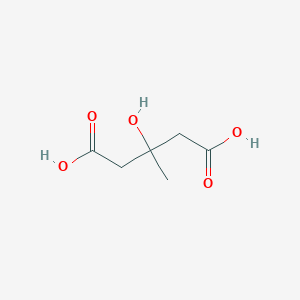
3-Hydroxymethylglutaric acid is an "off-product" intermediate in the leucine degradation process. It is produced by defective or inefficient versions of 3-hydroxy-3-methylglutaryl-CoA lyase, an enzyme that normally catalyzes the conversion of 3-hydroxy-3-methylglutaryl-CoA to acetyl-CoA and acetoacetate. If this enzyme is defective, 3-hydroxy-3-methylglutaryl-CoA will accumulate in the mitochondria. Increased concentrations of 3-hydroxy-3-methylglutaryl-CoA can lead to a disruption of the esterified CoA:free CoA ratio and ultimately to mitochondrial toxicity. Detoxification of these CoA end products occurs via the transfer of the 3-hydroxymethylglutaryl moiety to carnitine, forming 3-hydroxymethylglutaric-carnitine, which is then transferred across the inner mitochondrial membrane where 3-hydroxymethylglutaric acid is released as the free acid. 3-Hydroxymethylglutaric acid has been found to accumulate in the urine of patients affected by 3-Hydroxy-3-methylglutaric aciduria, a rare inborn error of metabolism (OMIM: 246450). 3-Hydroxy-3-methylglutaric aciduria is caused by significantly reduced enzyme activity of the intramitochondrial 3-hydroxy-3-methylglutaryl-CoA lyase (EC 4.1.3.4), the enzyme that catalyzes the final step of leucine degradation. This enzyme also plays a key role in ketone body formation. The profile of urinary organic acids for individuals with 3-hydroxy-3-methylglutaric aciduria is different from that of the other identified defects of leucine degradation, such as maple syrup urine disease (OMIM: 248600), isovaleric acidemia (OMIM: 243500), and methylcrotonylglycinemia (OMIM: 210200). The urinary organic acid profile of 3-hydroxy-3-methylglutaric aciduria includes elevated concentrations of 3-hydroxy-3-isovaleric, 3-hydroxy-3-methylglutaric, 3-methylglutaconic, and 3-methylglutaric acids. Clinical manifestations of 3-hydroxy-3-methylglutaric aciduria include hepatomegaly, lethargy, coma, and apnea. Biochemically, there is a characteristic absence of ketosis with hypoglycemia, acidosis, hypertransaminasemia, and variable hyperammonemia. Therefore, when present in sufficiently high concentrations, 3-hydroxymethylglutaric acid can act as an acidogen and a metabotoxin. An acidogen is an acidic compound that induces acidosis, which has multiple adverse effects on many organ systems. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. As noted above, chronically high levels of 3-hydroxymethylglutaric acid are associated with the inborn error of metabolism 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. 3-Hydroxymethylglutaric acid is an organic acid. Abnormally high levels of organic acids in the blood (organic acidemia), urine (organic aciduria), the brain, and other tissues lead to general metabolic acidosis. Acidosis typically occurs when arterial pH falls below 7.35. In infants with acidosis, the initial symptoms include poor feeding, vomiting, loss of appetite, weak muscle tone (hypotonia), and lack of energy (lethargy). These can progress to heart, liver, and kidney abnormalities, seizures, coma, and possibly death. These are also the characteristic symptoms of the untreated IEMs mentioned above. Many affected children with organic acidemias experience intellectual disability or delayed development. In adults, acidosis or acidemia is characterized by headaches, confusion, feeling tired, tremors, sleepiness, and seizures.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair