| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
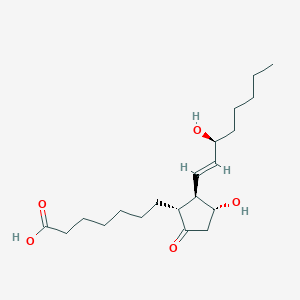
(+)-3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate; (+)-3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic acid; (-)-3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate; (-)-3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic acid; (-)-Prostaglandin e1; (11a,13E,15S)-11,15-Dihydroxy-9-oxoprost-13-en-1-Oate; (11a,13E,15S)-11,15-Dihydroxy-9-oxoprost-13-en-1-Oic acid; (11alpha,13E,15S)-11,15-Dihydroxy-9-oxoprost-13-en-1-Oic acid; (13E)-(15S)-11,15-Dihydroxy-9-oxoprost-13-enoate; (13E)-(15S)-11,15-Dihydroxy-9-oxoprost-13-enoic acid; (13E)-(15S)-11-alpha,15-Dihydroxy-9-oxoprost-13-enoate; (13E)-(15S)-11-alpha,15-Dihydroxy-9-oxoprost-13-enoic acid; (13E)-(15S)-11a,15-Dihydroxy-9-oxoprost-13-enoate; (13E)-(15S)-11a,15-Dihydroxy-9-oxoprost-13-enoic acid; (13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate; 11,15-Dihydroxy-9-oxoprost-13-en-1-Oate; 11,15-Dihydroxy-9-oxoprost-13-en-1-Oic acid; 11,15-Dihydroxy-9-oxoprost-13-en-1-Oic acid (acd/name 4.0); 11a,15a-Dihydroxy-9-oxo-13-trans-prostenoate; 11a,15a-Dihydroxy-9-oxo-13-trans-prostenoic acid; 11alpha,15alpha-Dihydroxy-9-oxo-13-trans-prostenoic acid; 3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate; 3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic acid; Abbott brand OF alprostadil; Allphar brand OF alprostadil; Alprostadil; Alprostadil prostoglandin e1; Alprostadil(usan); Alprostadilum; Astra brand OF alprostadil; AstraZeneca brand OF alprostadil; Befar; Caverject; Caverject impulse; Edex; Hoyer brand OF alprostadil; Janssen brand OF alprostadil; L-Prostaglandin e1; Lipo pge1; Lipo-pge1; Minprog; Muse; PGE-1; PGE1; PGE1alpha; Paladin brand OF alprostadil; Pharmacia brand 1 OF alprostadil; Pharmacia brand 2 OF alprostadil; Prink; Prostaglandin e1; Prostaglandin e1alpha; Prostavasin; Prostin VR; Prostin VR pediatric; Prostine VR; Schwarz brand OF alprostadil; Schwarz pharma brand OF alprostadil; Sugiran; U-10136alprostadil; U-10136prostin; Vasaprostan; Viridal; Vitaros; Vivus brand OF alprostadil
|
| Function |
Prostaglandin E1 (PGE1) is a potent endogenous vasodilator agent that increases peripheral blood flow. It inhibits platelet aggregation and has many other biological effects such as bronchodilation, mediation of inflammation, and various protective functions. The protective action of PGE1 has been shown on both experimental animal models of liver injury and patients with fulminant viral hepatitis. PGE1-treated cirrhotic rats had less hepatosplenomegaly, lower serum alanine aminotransferase levels and portal pressures, and higher arterial pressure than placebo-treated cirrhotic rats. There are several mechanisms of PGE1 hepatic cytoprotection: inhibiting T-cell mediated cytotoxicity, enhancing DNA synthesis of the injured liver after partial hepatectomy by stimulating cyclic AMP production, increasing ATP level in hepatic tissue to accelerate the recovery of mitochondrial respiratory function after reperfusion, and stabilizing membrane microviscosity. PGE1 is a prostanoid. The term prostanoid collectively describes prostaglandins, prostacyclins, and thromboxanes. Prostanoids are a subclass of the lipid mediator group known as eicosanoids. They are derived from C-20 polyunsaturated fatty acids, mainly dihomo--linolenic (20:3n-6), arachidonic (20:4n-6), and eicosapentaenoic (20:5n-3) acids, through the action of cyclooxygenases-1 and -2 (COX-1 and COX-2). Prostaglandins are eicosanoids. The eicosanoids consist of the prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and lipoxins (LXs). The PGs and TXs are collectively identified as prostanoids. Prostaglandins were originally shown to be synthesized in the prostate gland, thromboxanes from platelets (thrombocytes), and leukotrienes from leukocytes, hence the derivation of their names. All mammalian cells except erythrocytes synthesize eicosanoids. These molecules are extremely potent and are able to cause profound physiological effects at very dilute concentrations. All eicosanoids function locally at the site of synthesis through receptor-mediated G-protein linked signalling pathways.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair