| General Information of MET (ID: META00084) |
| Name |
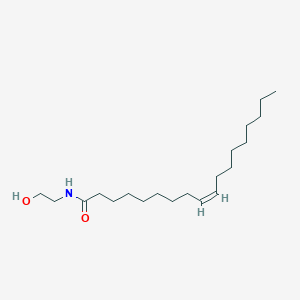
Oleoylethanolamide
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(9Z)-N-(2-Hydroxyethyl)-9-octadecenamide; N-(2-Hydroxyethyl)-9-Z-octadecenamide; N-(2-Hydroxyethyl)-9-octadecenamide; N-(2-Hydroxyethyl)oleamide; N-(9Z-Octadecenoyl) ethanolamine; N-(9Z-Octadecenoyl)-ethanolamine; N-(Hydroxyethyl)oleamide; N-(cis-9-Octadecenoyl) ethanolamine; N-OEA; N-Oleoyl ethanolamine; N-Oleoyl ethanolamine, oleoyl monoethanolamide, oleoylethanolamide; N-Oleoyl-2-aminoethanol; N-Oleoylethanolamide; N-Oleoylethanolamine; NOE; OEA; Oleamide mea; Oleic acid ethanolamide; Oleic acid monoethanolamide; Oleoyl 1-ethanolamide; Oleoyl ethanolamide; Oleoyl monoethanolamide; Oleoylethanolamide; Oleoylethanolamine
|
| Source |
Endogenous;Fatty acyls;Food;Cosmetic
|
| Structure Type |
Amines (Click to Show/Hide the Complete Structure Type Hierarchy)
Organic nitrogen compounds
Organonitrogen compounds
Amines
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C20H39NO2
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5283454"></iframe>
|
 |
|
3D MOL is unavailable
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
325.5 |
Topological Polar Surface Area |
49.3 |
| XlogP |
6.3 |
Complexity |
277 |
| Heavy Atom Count |
23 |
Rotatable Bond Count |
17 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Function |
Oleoylethanolamide (OEA or NOE) is an N-acylethanolamine. N-Acylethanolamines (NAEs) constitute a class of lipid compounds naturally present in both animal and plant membranes as constituents of the membrane-bound phospholipid, N-acylphosphatidylethanolamine (NAPE). NAPE is composed of a third fatty acid moiety linked to the amino head group of the commonly occurring membrane phospholipid, phosphatidylethanolamine. NAEs are released from NAPE by phospholipase D-type hydrolases in response to a variety of stimuli. Transient NAE release and accumulation have been attributed to a variety of biological activities, including neurotransmission, membrane protection, and immunomodulation in animals. Oleoylethanolamide is an inhibitor of the sphingolipid signalling pathway, via specific ceramidase inhibition (ceramidase converts ceramide to sphingosine). Oleoylethanolamide blocks the effects of TNF and arachidonic acid on intracellular Ca concentration. Oleoylethanolamide is related to the endocannabinoid anandamide. Endocannabinoids signal through cannabinoid receptors (also stimulated by the active ingredient of cannabis) but although related in structure, synthesis, and degradation to anandamide, OEA cannot be considered an endocannabinoid as it does not activate the cannabinoid receptors. Most of the reported responses to OEA can be attributed to the activation of peroxisome proliferator-activated receptor-alpha (PPAR-alpha). Administration of OEA inhibits body weight gain in rats. In adipocytes and hepatocytes, OEA inhibits mitogenic and metabolic signalling by the insulin receptor and produces glucose intolerance. It also inhibits gastric emptying, which might act together with the sensory neuronal signals to achieve satiety. OEA is permanently elevated in diabetic obese patients. OEA also reduces visceral and inflammatory responses through a PPAR-alpha-activation independent mechanism. OEA is an antagonist of TRVP1 (the transient receptor potential vanilloid type 1 receptor). Overall, OEA has beneficial effects on health by inducing food intake control, lipid beta-oxidation, body weight loss and analgesic effects.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

