|
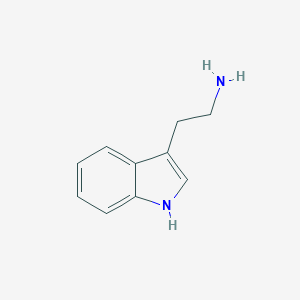
Tryptamine, also known as TrpN, is a catabolite of tryptophan converted by the gut microbiota. After absorption through the intestinal epithelium, tryptophan catabolites enter the bloodstream and are later excreted in the urine. Both Clostridium sp. and Ruminococcus sp. have been found to convert tryptophan into tryptamine. Tryptamine is a monoamine compound that is a common precursor molecule to many hormones and neurotransmitters. Biosynthesis generally proceeds from the amino acid tryptophan, with tryptamine acting as a precursor for other compounds. Substitutions to the tryptamine molecule give rise to a group of compounds collectively known as tryptamines. The most well-known tryptamines are serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine has been detected, but not quantified in, several different foods, such as onion-family vegetables, acerola, Japanese walnuts, custard apples, and green zucchinis. This could make tryptamine a potential biomarker for the consumption of these foods.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair