| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
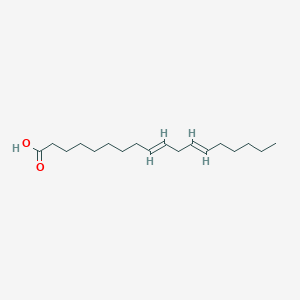
(9E,12E)-9,12-Octadecadienoate; (9E,12E)-9,12-Octadecadienoic acid; 18:2, N-6,9 all-trans; 9 trans,12 trans Octadecadienoic acid; 9,12 Octadecadienoic acid; 9,12-Octadecadienoic acid; 9-trans,12-trans-Octadecadienoic acid; 9E,12E-Octadecadienoate; 9E,12E-Octadecadienoic acid; Acid, 9,12-octadecadienoic; C18:2, N-6,9 all-trans; Linoelaidate; Linoelaidic acid, (e,Z)-isomer; Linoleate; Linoleic acid; Linoleic acid, (Z,Z)-isomer; Linoleic acid, (Z,Z)-isomer, 14C-labeled; Linoleic acid, (Z,e)-isomer; Linoleic acid, (e,e)-isomer; Linoleic acid, ammonium salt, (Z,Z)-isomer; Linoleic acid, calcium salt, (Z,Z)-isomer; Linoleic acid, potassium salt, (Z,Z)-isomer; Linoleic acid, sodium salt, (Z,Z)-isomer; Linoleic acid, sodium salt, (e,e)-isomer; Linolelaidic acid; cis,cis-9,12-Octadecadienoic acid; trans,trans-9,12-Octadecadienoic acid; trans-9,trans-12-Linoleate; trans-9,trans-12-Linoleic acid
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair