| General Information of MET (ID: META00823) |
| Name |
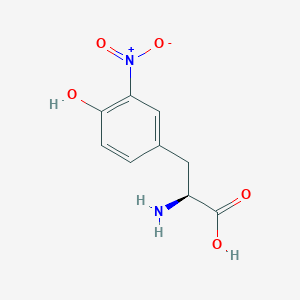
3-Nitrotyrosine
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(2S)-2-Amino-3-(4-hydroxy-3-nitrophenyl)propanoate; (2S)-2-Amino-3-(4-hydroxy-3-nitrophenyl)propanoic acid; 3-Mononitrotyrosine; 3-Nitrotyrosine; 3-Nitrotyrosine, (DL)-isomer; 3-Nitrotyrosine, (L)-isomer; 5-Nitrotyrosine; L-3-Nitrotyrosine; META-nitro-tyrosine; Nitro-tyrosine; Nitrotyrosine; m-Nitrotyrosine
|
| Source |
Endogenous;Food;Drug
|
| Structure Type |
Amino acids, peptides, and analogues (Click to Show/Hide the Complete Structure Type Hierarchy)
Organic acids and derivatives
Carboxylic acids and derivatives
Amino acids, peptides, and analogues
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C9H10N2O5
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=65124"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
226.19 |
Topological Polar Surface Area |
129 |
| XlogP |
-2 |
Complexity |
278 |
| Heavy Atom Count |
16 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
6 |
| Function |
3-Nitrotyrosine (NTyr) is formed in vivo in tissue or blood proteins after exposure to nitrosating and/or nitrating agents such as tetranitromethane. Reactive nitrogen species such as peroxynitrite can nitrate specific amino acids, whether free or protein bound, and 3-nitrotyrosine is believed to be one marker of this reaction.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

