| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
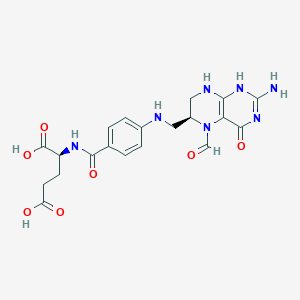
(6S)-5-Formyl-5,6,7,8-tetrahydrofolate; (6S)-5-Formyl-5,6,7,8-tetrahydrofolic acid; (6S)-5-Formyltetrahydrofolate; (6S)-5-Formyltetrahydrofolic acid; (6S)-Folinate; (6S)-Folinic acid; (6S)-Leucovorin; (S)-Leucovorin; 5 Formyltetrahydrofolate; 5 Formyltetrahydropteroylglutamate; 5-Formyl-(6S)-tetrahydrofolic acid; 5-Formyl-5,6,7,8-tetrahydrofolic acid; 5-Formyltetrahydrofolate; 5-Formyltetrahydrofolic acid; 5-Formyltetrahydropteroylglutamate; 5-Formyltetrahydropteroylglutamic acid; 6 S Leucovorin; 6-S-Leucovorin; 6S Leucovorin; 6S-Leucovorin; Acid, folinic; CF; Calcium folinate; Calcium leucovorin; Citrovorum factor; Factor, citrovorum; Folinate; Folinate, calcium; Folinic acid; Folinic acid SF; Folinic acid-SF; Fusilev; Kunyrin; L-Folinate; L-Folinic acid; Leucoverin; Leucovorin; Leucovorin, (D)-isomer; Leucovorin, (DL)-isomer; Leucovorin, (R)-isomer; Leucovorin, (S)-isomer; Leucovorin, calcium; Leucovorin, calcium (1:1) salt; Leucovorin, calcium (1:1) salt, (DL)-isomer; Leucovorin, calcium (1:1) salt, (S)-isomer; Leucovorin, calcium (1:1) salt, pentahydrate; Leucovorin, monopotassium salt, (S)-isomer; Leucovorin, monosodium salt; Leucovorin, monosodium salt, (S)-isomer; Leukovorin; Leukovorum; Levo leucovorin; Levo-leucovorin; Levofolene; Levofolinate; Levofolinic acid; Levoleucovorin; Monosodium salt leucovorin; N(5)-Formyltetrahydrofolate; N-[4-({[(6S)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzoyl]-L-glutamate; N-[4-({[(6S)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzoyl]-L-glutamic acid; N5-Formyl-5,6,7,8-tetrahydrofolic acid; N5-Formyl-THF; N5-Formyltetrahydrofolic acid; Pteroyl-D-glutamate; S Leucovorin; S-Leucovorin; Welcovorin; Wellcovorin
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair