| General Information of MET (ID: META00810) |
| Name |
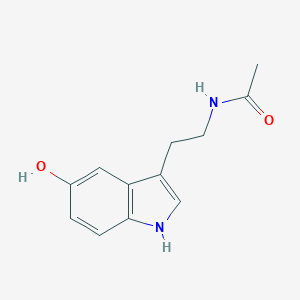
N-Acetylserotonin
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
5-Hydroxy-N-acetyltryptamine; 5-Hydroxymelatonin; ASE; Desmethylmelatonin; N-(2-(5-Hydroxy-1H-indol-3-yl)ethyl)acetamide; N-Acetyl-5-hydroxytryptamine; N-Acetylhydroxytryptamine; O-Demethylmelatonin
|
| Source |
Endogenous;Drug Metabolite;Food;Drug
|
| Structure Type |
Hydroxyindoles (Click to Show/Hide the Complete Structure Type Hierarchy)
Organoheterocyclic compounds
Indoles and derivatives
Hydroxyindoles
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C12H14N2O2
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=903"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
218.25 |
Topological Polar Surface Area |
65.099 |
| XlogP |
0.5 |
Complexity |
257 |
| Heavy Atom Count |
16 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
2 |
| Function |
N-Acetylserotonin is an intermediate in the metabolic pathway of melatonin and indoleamine in the pineal gland of mammalians. Serotonin-N-acetyltransferase (SNAT), which regulates the rate of melatonin biosynthesis in the pineal gland, catalyzes the acetylation of 5HT to N-acetylserotonin (NAS). A methyl group from S-adenosylmethionine is transferred to NAS by hydroxyindole-O-methyltransferase (HIOMT), and finally NAS is converted to 5-methoxy-N-acetyltryptamine, or melatonin. In most mammalian species the content of NAS (and melatonin) in the pineal gland shows clear circadian changes with the highest level occurring during the dark period. This elevation of the contents of NAS (and melatonin) in the dark period is due to the increase of SNAT activity and the elevation of SNAT gene expression. Experimental studies show that N-acetylserotonin possess free radical scavenging activity. Acute administration of irreversible and reversible selective MAO-A inhibitors and high doses (or chronic administration of low doses) of relatively selective MAO-B inhibitors (but not of highly selective MAO-B inhibitors) suppressed MAO-A activity and stimulated N-acetylation of pineal serotonin into N-acetylserotonin, the immediate precursor of melatonin. N-acetylserotonin increase after MAO-A inhibitors might mediate their antidepressive and antihypertensive effects. N-Acetylserotonin is the product of the O-demethylation of melatonin mediated by cytochrome P-450 isoforms: Cytochrome p450, subfamily IIc, polypeptide 19 (CYP2C19, a clinically important enzyme that metabolizes a wide variety of drugs), with a minor contribution from Cytochrome p450, subfamily I, polypeptide (2CYP1A2, involved in O-deethylation of phenacetin).
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

