| General Information of MET (ID: META00796) |
| Name |
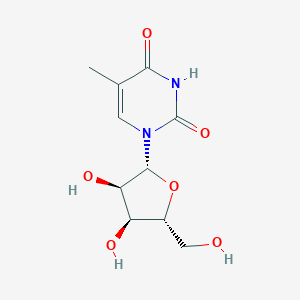
Ribothymidine
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
1-(beta-D-Ribofuranosyl)thymine; 1-b-D-Ribofuranosylthymine; 1-beta-delta-Ribofuranosylthymine; 5-Methyl-1-beta-D-ribofuranosyl-2,4(1H,3H)-pyrimidinedione; 5-Methyl-1-beta-delta-ribofuranosyl-2,4(1H,3H)-pyrimidinedione; 5-Methyl-uridine; 5-Methyluridine; Ribosylthymidine; Ribosylthymine; Thymine ribofuranoside; Thymine ribonucleoside; Thymine riboside; Thymine-1 beta-D-ribofuranosylthymine; Thymine-1 beta-delta-ribofuranosylthymine; b-D-Ribofuranoside thymine-1; beta-D-Ribofuranoside; beta-delta-Ribofuranoside; beta-delta-Ribofuranoside thymine-1; t
|
| Source |
Endogenous;Escherichia Coli Metabolite;Food;Microbial
|
| Structure Type |
Pyrimidine nucleosides (Click to Show/Hide the Complete Structure Type Hierarchy)
Nucleosides, nucleotides, and analogues
Pyrimidine nucleosides
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C10H14N2O6
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=445408"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
258.23 |
Topological Polar Surface Area |
119 |
| XlogP |
-1.6 |
Complexity |
409 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
6 |
| Function |
Ribothymidine is an endogenous methylated nucleoside found in human fluids; methylated purine bases are present in higher amounts in tumor-bearing patients compared to healthy controls.DNA hypermethylation is a common finding in malignant cells and has been explored as a therapeutic target for hypomethylating agents. When chemical bonds to DNA, the DNA becomes damaged and proper and complete replication cannot occur to make the normal intended cell. A DNA adduct is an abnormal piece of DNA covalently-bonded to a cancer-causing chemical. This has shown to be the start of a cancerous cell, or carcinogenesis. DNA adducts in scientific experiments are used as bio-markers and as such are themselves measured to reflect quantitatively, for comparison, the amount of cancer in the subject.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

