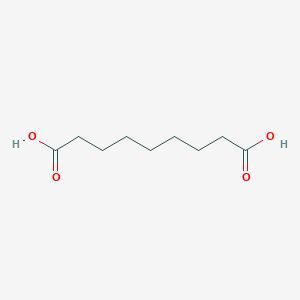
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
1,7-Dicarboxyheptane; 1,7-Heptanedicarboxylate; 1,7-Heptanedicarboxylic acid; 1,9-Nonanedioate; 1,9-Nonanedioic acid; Acide azelaique; Acidum azelaicum; Anchoate; Anchoic acid; Azalaic acid; Azelaate; Azelaic acid, dilithium salt; Azelaic acid, dipotassium salt; Azelaic acid, disodium salt; Azelaic acid, monosodium salt; Azelaic acid, potassium salt; Azelaic acid, sodium salt; Azelaicacidtech; Azelainic acid; Azelainsaeure; Azelate; Azelex; Emerox 1110; Emerox 1144', Emery'S L-110, 'Finevin; Finacea; Heptanedicarboxylic acid; Lepargylate; Lepargylic acid; Monosodium azelate; N-Nonanedioate; N-Nonanedioic acid; Nonandisaeure; Nonanedioate; Nonanedioic acid; Nonanedioic acid azelaic acid; Nonanedioic acid homopolymer; Poly(azelaic anhydride); Polyazelaic anhydride; Skinorem; Skinoren
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair