|
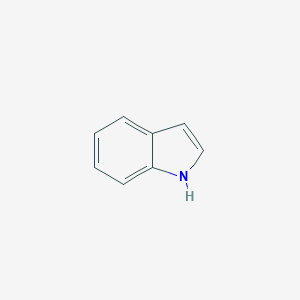
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. The participation of the nitrogen lone electron pair in the aromatic ring means that indole is not a base, and it does not behave like a simple amine. Indole is a microbial metabolite and it can be produced by bacteria as a degradation product of the amino acid tryptophan. It occurs naturally in human feces and has an intense fecal smell. At very low concentrations, however, indole has a flowery smell and is a constituent of many flower scents (such as orange blossoms) and perfumes. As a volatile organic compound, indole has been identified as a fecal biomarker of Clostridium difficile infection. Natural jasmine oil, used in the perfume industry, contains around 2.5% of indole. Indole also occurs in coal tar. Indole has been found to be produced in a number of bacterial genera including Alcaligenes, Aspergillus, Escherichia, and Pseudomonas. Indole plays a role in bacterial biofilm formation, bacterial motility, bacterial virulence, plasmid stability, and antibiotic resistance. It also functions as an intercellular signalling molecule. Recently, it was determined that the bacterial membrane-bound histidine sensor kinase (HK) known as CpxA acts as a bacterial indole sensor to facilitate signalling. It has been found that decreased indole concentrations in the gut promote bacterial pathogenesis, while increased levels of indole in the gut decrease bacterial virulence gene expression. As a result, enteric pathogens sense a gradient of indole concentrations in the gut to migrate to different niches and successfully establish an infection.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair