| General Information of MET (ID: META00777) |
| Name |
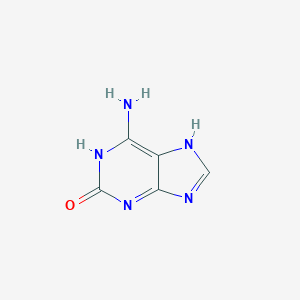
2-Hydroxyadenine
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
2-Hydroxy-6-aminopurine; 2-Hydroxyadenine; 2-OH-Ade; 2-Oxoadenine; 2-Oxyadenine; 6-Amino-1,3-dihydro-2H-purin-2-one; 6-Amino-2-hydroxypurine; 6-Amino-3,7(9)-dihydro-purin-2-one; 6-Amino-3,7-dihydro-purin-2-one; 6-Amino-3,9-dihydro-2H-purin-2-one; 6-Amino-7H-purin-2-ol; 6-Amino-9H-purin-2-ol; Crotonoside; Isoguanine
|
| Source |
Endogenous;Food;TCM Ingredients
|
| Structure Type |
Purines and purine derivatives (Click to Show/Hide the Complete Structure Type Hierarchy)
Organoheterocyclic compounds
Imidazopyrimidines
Purines and purine derivatives
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C5H5N5O
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=76900"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
151.13 |
Topological Polar Surface Area |
91.9 |
| XlogP |
-1.7 |
Complexity |
313 |
| Heavy Atom Count |
11 |
Rotatable Bond Count |
N.A. |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
2 |
| Function |
2-Hydroxyadenine (2-OH-Ade) is formed by hydroxyl radical attack on DNA bases and shows a genotoxicity in human, being the source of the mutations induced by reactive oxygen species. 2-OH-Ade in DNA is miscoding and elicits various mutations, and is a mutagenic in bacterial and mammalian cells. (Recent Research Developments in Biochemistry (2000)2:41-50).
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

