| General Information of MET (ID: META00771) |
| Name |
L-Palmitoylcarnitine
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
(+)-Palmitoylcarnitine; (2R)-Palmitoylcarnitine; (3R)-3-(Hexadecanoyloxy)-4-(trimethylazaniumyl)butanoate; (3R)-3-(Hexadecanoyloxy)-4-(trimethylazaniumyl)butanoic acid; (3R)-3-Palmitoyloxy-4-(trimethylammonio)butanoate; (3R)-3-Palmitoyloxy-4-(trimethylammonio)butanoic acid; 3-Carboxy-N,N,N-trimethyl-2-[(1-oxohexadecyl)oxy]-1-propanaminium; C16 Carnitine; Hexadecanoyl-L-carnitine; Hexadecanoylcarnitine; Hexadecenoyl carnitine; L(-)-Palmitylcarnitine; L-Carnitine palmitoyl ester; L-Palmitoyl-L-carnitine; L-Palmitoylcarnitine; O-Hexadecanoyl-(R)-carnitine; O-Hexadecanoyl-R-carnitine; Palmitoyl-(-)-carnitine; Palmitoyl-L-carnitine; Palmitoylcarnitine; Palmityl-L-carnitine; Palmitylcarnitine
|
| Source |
Aliphatic acyclic compounds
|
| Structure Type |
Fatty acid esters (Click to Show/Hide the Complete Structure Type Hierarchy)
Lipids and lipid-like molecules
Fatty Acyls
Fatty acid esters
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C23H45NO4
|
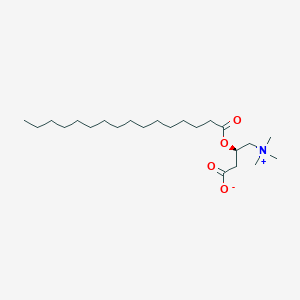
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=11953816"></iframe>
|
 |
|
3D MOL is unavailable
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
399.6 |
Topological Polar Surface Area |
66.4 |
| XlogP |
7.7 |
Complexity |
398 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
19 |
| Hydrogen Bond Donor Count |
N.A. |
Hydrogen Bond Acceptor Count |
4 |
| Function |
L-Palmitoylcarnitine is a long-chain acyl fatty acid derivative ester of carnitine which facilitates the transfer of long-chain fatty acids from cytoplasm into mitochondria during the oxidation of fatty acids. L-Palmitoylcarnitine, due to its amphipathic character is, like detergents, a surface-active molecule. By changing the membrane fluidity and surface charge they can change the activity of several enzymes and transporters localized in the membrane. L-Palmitoylcarnitine has been also reported to change the activity of certain proteins. On the contrary to carnitine, palmitoylcarnitine was shown to stimulate the activity of caspases 3, 7, and 8 and the level of this long-chain acylcarnitine increased during apoptosis. Palmitoylcarnitine was also reported to diminish the binding of phorbol esters, the protein kinase C activators, and the autophosphorylation of the enzyme. Apart from these isoform nonspecific phenomena, palmitoylcarnitine was also shown to be responsible for retardation in the cytoplasm of protein kinase C isoforms and and, in the case of the latter one, to decrease its interaction with GAP-43. Some of the physicochemical properties of palmitoylcarnitine may help to explain the need for coenzyme A-carnitine-coenzyme A acyl exchange during mitochondrial fatty acid import. The amphiphilic character of palmitoylcarnitine may also explain its proposed involvement in the pathogenesis of myocardial ischemia. L-Palmitoylcarnitine accumulates in ischemic myocardium and potentially contributes to myocardial damage through alterations in membrane molecular dynamics. This is a mechanism through which could play an important role in ischemic injury. Palmitoylcarnitine is characteristically elevated in carnitine palmitoyltransferase II deficiency, late-onset (OMIM: 255110). Moreover, L-palmitoylcarnitine is found to be associated with celiac disease, which is an inborn error of metabolism.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

