| General Information of MET (ID: META00595) |
| Name |
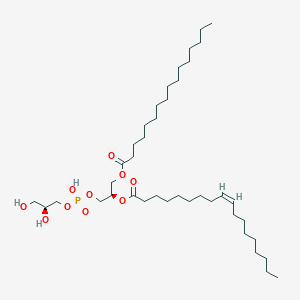
PG(16:0/18:1(9Z))
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-sn-glycerol', 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-sn-glycerol), '1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol; 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoglycerol; 16:0/18:1 PG; C16:0/18:1 PG; GPG(16:0/18:1(9Z)); GPG(16:0/18:1); GPG(16:0/18:1OMEGA9); GPG(16:0/18:1W9); GPG(34:1); PG(16:0/18:1(9Z)); PG(16:0/18:1); PG(16:0/18:1N9); PG(16:0/18:1OMEGA9); PG(16:0/18:1W9); PG(34:1); Phosphatidylglycerol(16:0/18:1(9Z)); Phosphatidylglycerol(16:0/18:1); Phosphatidylglycerol(16:0/18:1W9); Phosphatidylglycerol(16:0/18:1n9); Phosphatidylglycerol(16:0/18:1omega9); Phosphatidylglycerol(34:1)', 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-(1'-glycerol), 'GPG(16:0/18:1N9)
|
| Source |
Endogenous;Escherichia Coli Metabolite;Yeast Metabolite;Glycerophospholipids;Food
|
| Structure Type |
Glycerophosphoglycerols (Click to Show/Hide the Complete Structure Type Hierarchy)
Lipids and lipid-like molecules
Glycerophospholipids
Glycerophosphoglycerols
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C40H77O10P
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=52941750"></iframe>
|
 |
|
3D MOL is unavailable
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| Physicochemical Properties |
Molecular Weight |
749 |
Topological Polar Surface Area |
149 |
| XlogP |
12.3 |
Complexity |
868 |
| Heavy Atom Count |
51 |
Rotatable Bond Count |
41 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
10 |
| Function |
PG(16:0/18:1(9Z)) is a phosphatidylglycerol or glycerophospholipid (PG or GP). It is a glycerophospholipid in which a phosphoglycerol moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PG(16:0/18:1(9Z)), in particular, consists of one chain of palmitic acid at the C-1 position and one chain of oleic acid at the C-2 position. The palmitic acid moiety is derived from fish oils, milk fats, vegetable oils and animal fats, while the oleic acid moiety is derived from vegetable oils, especially olive and canola oil. Phosphatidylglycerol is present at a level of 1-2% in most animal tissues, but it can be the second most abundant phospholipid in lung surfactant at up to 11% of the total. It is well established that the concentration of phosphatidylglycerol increases during fetal development. Phosphatidylglycerol may be present in animal tissues merely as a precursor for diphosphatidylglycerol (cardiolipin). Phosphatidylglycerol is formed from phosphatidic acid by a sequence of enzymatic reactions that proceeds via the intermediate, cytidine diphosphate diacylglycerol (CDP-diacylglycerol). Bioynthesis proceeds by condensation of phosphatidic acid and cytidine triphosphate with elimination of pyrophosphate via the action of phosphatidate cytidyltransferase (or CDP-synthase). CDP-diacylglycerol then reacts with glycerol-3-phosphate via phosphatidylglycerophosphate synthase to form 3-sn-phosphatidyl-1'-sn-glycerol 3'-phosphoric acid, with the release of cytidine monophosphate (CMP). Finally, phosphatidylglycerol is formed by the action of specific phosphatases. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PGs have a net charge of -1 at physiological pH and are found in high concentration in mitochondrial membranes and as components of pulmonary surfactant. PG also serves as a precursor for the synthesis of cardiolipin. PG is synthesized from CDP-diacylglycerol and glycerol-3-phosphate.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

