|
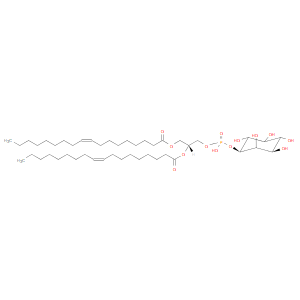
PI(18:1(9Z)/18:1(9Z)) is a phosphatidylinositol. Phosphatidylinositols are important lipids, both as a key membrane constituent and as a participant in essential metabolic processes, both directly and via a number of metabolites. Phosphatidylinositols are acidic (anionic) phospholipids that consist of a phosphatidic acid backbone, linked via the phosphate group to inositol (hexahydroxycyclohexane). Phosphatidylinositols can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 18 and 20 carbons are the most common. PI(18:1(9Z)/18:1(9Z)), in particular, consists of one chain of oleic acid at the C-1 position and one chain of oleic acid at the C-2 position. The oleic acid moiety is derived from vegetable oils, especially olive and canola oil, while the oleic acid moiety is derived from vegetable oils, especially olive and canola oil. The inositol group that is part of every phosphatidylinositol lipid is covalently linked to the phosphate group that acts as a bridge to the lipid tail. In most organisms, the stereochemical form of this inositol is myo-D-inositol (with one axial hydroxyl in position 2 with the remainder equatorial), although other forms can be found in certain plant phosphatidylinositols. Phosphatidylinositol is especially abundant in brain tissue, where it can amount to 10% of the phospholipids, but it is present in all tissues and cell types. There is usually less of it than of phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine. In animal tissues, phosphatidylinositol is the primary source of the arachidonic acid required for biosynthesis of eicosanoids, including prostaglandins, via the action of the enzyme phospholipase A2. Phosphatidylinositol can be phosphorylated by a number of different kinases that place the phosphate moiety on positions 4 and 5 of the inositol ring, although position 3 can also be phosphorylated by a specific kinase. Seven different isomers are known, but the most important in both quantitative and biological terms are phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate. Phosphatidylinositol and the phosphatidylinositol phosphates are the main source of diacylglycerols that serve as signaling molecules, via the action of phospholipase C enzymes.While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PIs contain almost exclusively stearic acid at carbon 1 and arachidonic acid at carbon 2. PIs composed exclusively of non-phosphorylated inositol exhibit a net charge of -1 at physiological pH. Molecules with phosphorylated inositol (such as PIP, PIP2, PIP3, etc.) are termed polyphosphoinositides. The polyphosphoinositides are important intracellular transducers of signals emanating from the plasma membrane. The synthesis of PI involves CDP-activated 1,2-diacylglycerol condensation with myo-inositol.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair