| General Information of MET (ID: META00437) |
| Name |
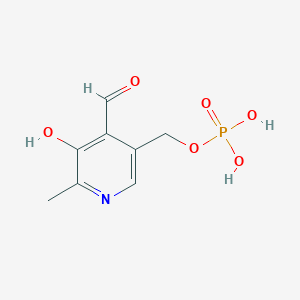
Pyridoxal 5'-phosphate
|
| Synonyms |
Click to Show/Hide Synonyms of This Metabolite
3-Hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde; 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-phosphate; Biosechs; Codecarboxylase; Coenzyme b6; Hairoxal; Hexermin-p; Hi-pyridoxin; Hiadelon; Himitan; PAL-p; PLP', Pyridoxal 5'-(dihydrogen phosphate), 'Pyridoxal 5-monophosphoric acid ester; Phosphate mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester', Pyridoxal 5'-(dihydrogen phosphoric acid), 'Pyridoxal 5-monophosphate ester; Phosphate, pyridoxal; Phosphopyridoxal; Phosphopyridoxal coenzyme; Phosphoric acid mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester; Pidopidon; Piodel; Pydoxal; Pyridoxal 5 phosphate; Pyridoxal 5-phosphate; Pyridoxal 5-phosphoric acid; Pyridoxal p; Pyridoxal phosphate', PYRIDOXAL-5'-phosphATE, '3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-phosphoric acid; Pyridoxal phosphoric acid', PYRIDOXAL-5'-phosphoric acid, Pyridoxal 5'-phosphoric acid, 'Apolon b6; Pyridoxal-p; Pyridoxyl phosphate; Pyromijin; Sechvitan; Vitahexin-p; Vitazechs
|
| Source |
Endogenous;Escherichia Coli Metabolite;Yeast Metabolite;Food;Drug;Cosmetic;Microbial
|
| Structure Type |
Pyridine carboxaldehydes (Click to Show/Hide the Complete Structure Type Hierarchy)
Organoheterocyclic compounds
Pyridines and derivatives
Pyridine carboxaldehydes
|
| PubChem CID |
|
| HMDB ID |
|
| Formula |
C8H10NO6P
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=1051"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
|
Click to Show/Hide the Molecular/Functional Data (External Links/Property/Function) of This Metabolite
|
| KEGG ID |
|
| DrugBank ID |
|
| ChEBI ID |
|
| FooDB ID |
|
| ChemSpider ID |
|
| METLIN ID |
|
| Physicochemical Properties |
Molecular Weight |
247.14 |
Topological Polar Surface Area |
117 |
| XlogP |
-1.1 |
Complexity |
292 |
| Heavy Atom Count |
16 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
7 |
| Function |
This is the active form of vitamin B6 serving as a coenzyme for synthesis of amino acids, neurotransmitters (serotonin, norepinephrine), sphingolipids, aminolevulinic acid. During transamination of amino acids, pyridoxal phosphate is transiently converted into pyridoxamine phosphate (pyridoxamine). -- Pubchem; Pyridoxal-phosphate (PLP, pyridoxal-5'-phosphate) is a cofactor of many enzymatic reactions. It is the active form of vitamin B6 which comprises three natural organic compounds, pyridoxal, pyridoxamine and pyridoxine. -- Wikipedia.
|
|
Regulatory Network
|
|
|
|
|
|
|
|
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair

