|
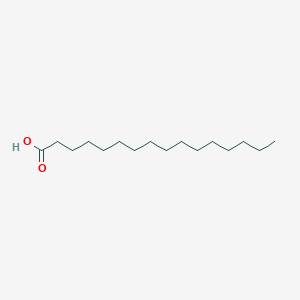
Palmitic acid, or hexadecanoic acid, is one of the most common saturated fatty acids found in animals, plants, and microorganisms. As its name indicates, it is a major component of the oil from the fruit of oil palms (palm oil). Excess carbohydrates in the body are converted to palmitic acid. Palmitic acid is the first fatty acid produced during fatty acid synthesis and is the precursor to longer fatty acids. As a consequence, palmitic acid is a major body component of animals. In humans, one analysis found it to make up 21-30% (molar) of human depot fat , and it is a major, but highly variable, lipid component of human breast milk. Palmitic acid is used to produce soaps, cosmetics, and industrial mould release agents. These applications use sodium palmitate, which is commonly obtained by saponification of palm oil. To this end, palm oil, rendered from palm tree (species Elaeis guineensis), is treated with sodium hydroxide (in the form of caustic soda or lye), which causes hydrolysis of the ester groups, yielding glycerol and sodium palmitate. Aluminium salts of palmitic acid and naphthenic acid were combined during World War II to produce napalm. The word "napalm" is derived from the words naphthenic acid and palmitic acid. Palmitic acid is also used in the determination of water hardness and is a surfactant of Levovist, an intravenous ultrasonic contrast agent.
|
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair
 click to show the details of this protein
click to show the details of this protein
 click to show the details of experiment for validating this pair
click to show the details of experiment for validating this pair